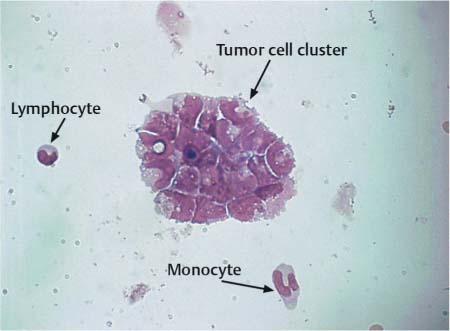
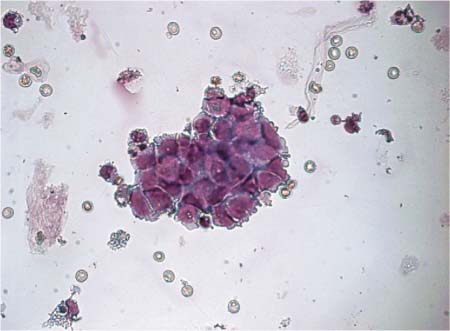
Fig. 16.1 a–c Primary melanosarcoma of the leptomeninges.
a Amelanotic tumor cells.
b Tumor cells with melanin deposition.
c Melanin-containing cells.
Fig. 16.2 Tumor dissemination: tumor cell cluster from a medulloblastoma.
In most cases, analysis of lumbar CSF is of much greater diagnostic value than analysis of ventricular CSF (Gajjar et al., 1999).
Cytology
Cell counts. Cell counts vary greatly:
• In glioblastoma, astrocytoma, and oligodendroglioma, cell counts are often normal or only slightly increased. Leptomeningeal spread is only expected in stage III and anaplastic tumors.
• In medulloblastoma (Fig. 16.2), germinoma and pinealoblastoma (Fig. 16.3), cell counts are often increased to several hundred cells/μL. The tumor cells are solitary or in clusters.
Morphology. In malignant tumors, undifferentiated polymorphic cells with large polymorphic nuclei and nucleoli are prominent. In germinoma, the cells may look like small lymphocytes or large, pale lymphoid cells. In ependymoma, the tumor cells are often difficult to differentiate from normal ependymal cells, choroid plexus cells, or mononuclear cells. Ependymoma cells are often large and occur in epithelioid clusters, showing a weakly gray-blue staining, foamy cytoplasm; their nuclei are rather small, sometimes pyknotic. Cells of benign choroid plexus papilloma look like normal plexus cells, and those of polymorphic malignant choroid plexus papilloma often appear in clusters and resemble cells of epithelial tumors.
Immunocytochemistry. Immunocytochemical differentiation enables the distinction between choroid plexus tumor cells, which express cytokeratin, and ependymoma cells, which express vimentin and glial fibrillary acidic protein (GFAP). Glioma cells do not differ immunocytochemically from ependymoma cells. Medulloblastoma cells are positive for GFAP and neurofilament (Gupta et al., 2004).
Fig. 16.3 Tumor dissemination: tumor cell cluster from a pinealoblastoma.
Tumor cells are often accompanied by an inflammatory reaction, a sign of a nonspecific defense reaction that may push the tumor cells into the background. In this case mononuclear cells usually predominate, often showing clear signs of lymphocyte transformation and activation. Plasma cells or granulocytes may also be present.
Neurochemistry
Total protein, lactate, NSE, and S 100. Total protein levels are rarely within the normal range. In most cases, a moderate to severe blood–CSF barrier dysfunction is found, with QAlb > 10. Pronounced increases in total protein are found in patients with intracranial and spinal neurinomas. Intrathecal immunoglobulin production, predominantly of IgG and IgM, is found in about 20%. Lactate levels are often elevated. Neuron-specific enolase (NSE) and S 100 protein are only nonspecific destruction markers and a sign of glial activation, so measuring them is not important in the diagnosis of primary brain tumors.
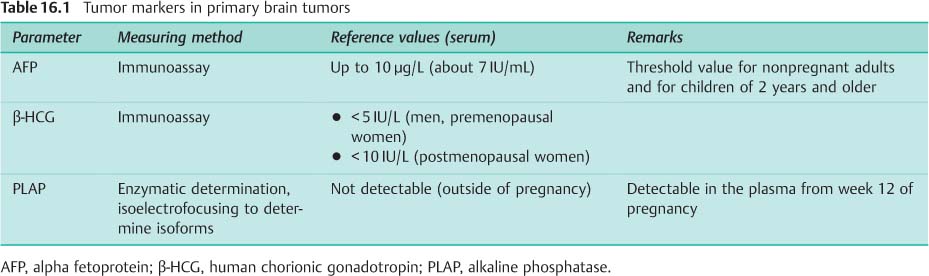
Tumor markers. As with extracerebral germ cell tumors, tumor markers are also detected in patients with intracranial tumors (Table 16.1). The values are usually higher in CSF than in serum taken at the same time:
• Alpha fetoprotein (AFP) is always elevated in embryonic carcinoma and yolk sac tumor.
• In both these tumors, an increase in the beta subunit of human chorionic gonadotropin (β-HCG) is also often found. In chorionic carcinoma the rise in β-HCG is always marked, in germinoma it is only slight.
• LDH values are frequently elevated in germinoma.
• In all these tumors placental alkaline phosphatase (PLAP) may be normal or elevated; measurement of this parameter has therefore declined in importance (Fleischhack and Bode, 2003).
Serum Analysis
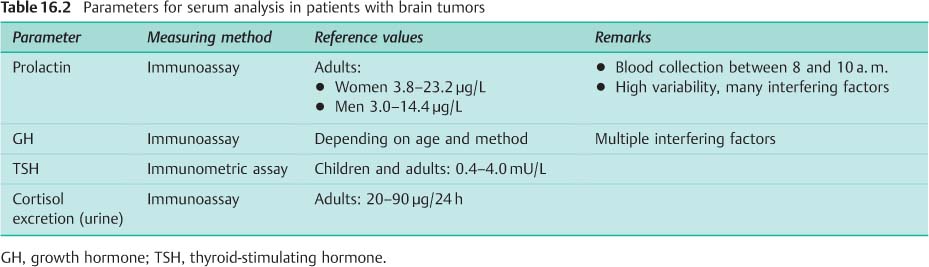
Clinically, 20–55% of hypophyseal adenomas are assessed as hormonally inactive. However, subtle test methods can detect hormone production in 20–40% of these patients. The basic diagnostic analysis for hypophyseal adenoma involves measuring prolactin, growth hormone, TSH, and cortisol (Table 16.2):
• Prolactin: Raised values are not always a sign of prolactinoma. Increased prolactin secretion can also be due to disordered hypothalamic dopamine secretion from another cause, to intake of various pharmaceutical drugs, to kidney or liver insufficiency, or to stress.
• Growth hormone: The production of growth hormone (GH) fluctuates greatly; a single GH value is therefore not informative. A suppression test is used, because normally GH secretion can be suppressed by glucose administration. If suppression does not occur, this indicates autonomic secretion of GH.
• Cortisol: Because cortisol production is subject to significant circadian fluctuations, a single cortisol value is not sufficient. The best screening test is determination of cortisol excretion in 24-hour urine.
Secondary Brain Tumors
Secondary brain tumors, or CNS metastases, represent disseminations of a systemic tumor in the central and/or peripheral nervous system, usually at an advanced stage of the tumor (Posner, 1995; Schackert and Schlegel, 2003). Although the improved treatment options for systemic tumors have increased survival times, they also result in an increase in solid brain metastases and/or leptomeningeal spread because the blood–brain barrier patency to many cytostatic drugs is poor.
Types. The following types of secondary tumors are distinguished:
• Space-occupying lesions in the brain parenchyma; confirmation of the diagnosis by detection of tumor cells in the CSF is usually only successful when the lesion is close to a ventricle.
• Invasion of the meninges by diffuse and/or nodular leptomeningeal metastasis.
Of the two types, leptomeningeal spread plays by far the more important role in CSF analysis.
Meningeal carcinomatosis. The highest incidence of carcinomatous infiltration of the meninges is from breast cancer (40–50%), followed by lung cancer (20–25%) and malignant melanoma (10%). Meningeal dissemination from gastrointestinal and urogenital carcinoma is rare (about 5%). In terms of pathogenesis, leptomeningeal spread is caused by local meningeal infiltration from epidural, osseous, cranial, or vertebral structures, or by hematogenous dissemination into the subarachnoid space.
Diagnosis. Leptomeningeal infiltration is usually suspected because of clinical symptoms (headache, personality and mental changes, impaired alertness, focal and/or generalized convulsions, cranial nerve dysfunction, and/or radicular symptoms) and substantiated by contrast enhancement of the leptomeninges on MRI. The diagnosis is confirmed by detection of tumor cells in the CSF (Kaplan et al., 1990).
Detection of tumor cells in the CSF confirms the diagnosis of leptomeningeal spread.
Cytology
Cell counts.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree