Fig. 9.1
The waveform of the BAEP can be recorded in three different montages, through the use of just three electrodes. Peaks I through to V are visible on the ipsilateral recording and II–V on the contralateral recording. Recordings from the two earlobes also allow all five peaks to be seen. The figure usefully illustrates the comparative ease of distinguishing different peaks depending on the montage selected. In practice, all of these can be easily run at the same time (from the 5th edition of Aminoff. Figure 23.6)
Tumors of CN VIII are known by a number of names, acoustic neuroma, vestibular schwannoma, and vestibular neuroma. For the most part, these tumors are derived from Schwann cells on the vestibular branch of CN VIII, so a vestibular schwannoma is possibly the best description [7]. Rarely the same tumor type can occur on the auditory portion of the nerve. These tumors are generally benign and slow growing. Many cases are treated with gamma knife technology as an alternative to surgery. Because both the vestibular portion and the auditory portion of CN VIII run so closely together for most of their length, monitoring of the BAEP is indicated in any tumor resection of CN VIII if the intent is to preserve hearing. Since posterior fossa craniotomy also places the brainstem at risk, the BAEP is also monitored as a way to detect brainstem ischemia. Bilateral BAEPs should always be recorded when possible. Although it is ideal for the IOM clinician to be able to participate in preoperative planning of these surgeries, in some instances this is not possible. If you are able to be part of the team preoperatively, it is helpful to know if there is any serviceable hearing left and to what degree. The patient’s facial nerve function can also be documented at this time since facial nerve monitoring will be performed during this type of case as well. In non-hearing preservation surgery, there is of course no need to monitor the BAEP ipsilaterally.
Microvascular decompressions (MVDs) for a number of conditions also can pose a risk to CN VIII, either directly or through ischemic changes. MVD of CN VIII is indicated in cases where the patient suffers from either disabling tinnitus (auditory portion) or positional vertigo (vestibular portion). BAEP monitoring should also be considered for MVD procedures to relieve trigeminal neuralgia (CN V), hemifacial spasm (CN VII), or glossopharyngeal neuralgia (CN IX) [1, 7–9].
Space-occupying lesions of the fourth ventricle can disrupt brainstem function. Because of its many relay stations within the brainstem (it is called the brainstem auditory evoked potential after all!), the BAEP is a useful monitor of brainstem health. However, it is important to remember that the brainstem performs a wonderful variety of neural functions and the BAEP only directly assesses a small function of the brainstem [10].
Tumors of the cerebellar pontine angle (CPA tumors) remain the most often indication for BAEP monitoring. Surgery to remove these tumors requires the neuromonitorist to bring their full armamentarium to the case, and that will undoubtedly involve the BAEP as well as EMG monitoring of the lower cranial nerves [11].
Peaks, Generators, and Blood Supply
The BAEP has five peaks or waves that are monitored in the intraoperative setting (Fig. 9.2) [12–14]. The peaks are named peak I through to peak V (wave is often substituted for peak). Peak I is generated in the distal auditory nerve, and there is very little controversy about the origin of peak I. However, for the rest of the peaks, the situation is a little more complicated as each peak has more than one potential generator. We will initially consider the primary generators or at least the generators that are most commonly considered the primary generators. Peak II is generated from the auditory nerve, but in this case the intracranial portion is also called the proximal portion of the auditory nerve. The third peak, peak III, is the first that originates from the secondary neurons, meaning that they are the first peaks for which a synapse is involved. The caudal pontine tegmentum is the principal generator of peak III, as well as the negative peak between peak III and peak IV, sometimes known as IIIa. There is some evidence however that there is a contribution from the cochlear nucleus to peak III as well as a contribution from the ascending activity within the lateral lemniscus. Peak III is usually not altered in individuals with lesions in the upper or middle pons, or even the mesencephalon, which is evidence that the generator lies caudal to this point. The most likely generator for peak IV is the SOC, but there is no conclusive evidence to date for a precise origin to be determined. The lateral lemniscus remains a candidate for the generator of peak IV. Peak V, the last of the BAEP peaks, is generated predominantly by the contralateral mesencephalon, specifically the inferior colliculus. The lesions of the pons and mesencephalon frequently affect peak V first in the time course of the disease, and as such, this peak may be abnormal in patients undergoing surgery even when the tumor is considered to be relatively small [13]. Later peaks are not considered as part of the BAEP for the purposes of IOM at present.
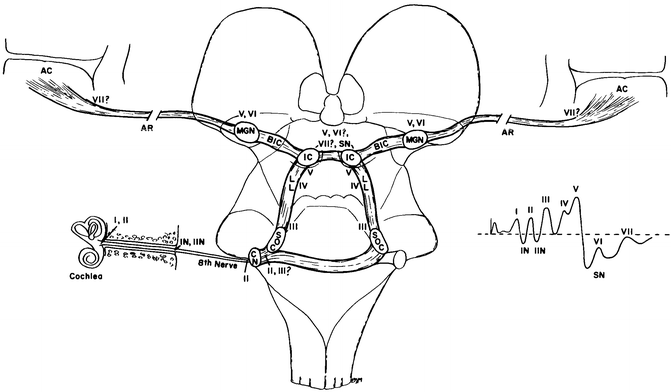

Fig. 9.2
The likely generators within the brainstem of auditory evoked potentials. The roman numerals refer to the individual peaks within the potential. CN cochlear nucleus, SOC superior olivary complex, LL lateral lemniscus, IC inferior colliculus, BIC brachium of the inferior colliculus, MGN medial geniculate nucleus, AR auditory radiations leading to AC, auditory cortex (from the 5th edition of Aminoff. Figure 23.16)
Preoperative Considerations
Preoperatively, the neuromonitorist must determine the baseline hearing of the patient. Often audiologists assess this formally before the surgical procedure is planned. An appropriate stimulation level for intraoperative BAEPs can then be determined. Preoperative BAEPs may be helpful if there is time to obtain one. This is one of the easier evoked potentials to perform on an awake patient. Very few people find the process uncomfortable [3, 15]. Gathering these data before the surgery may give insight into any apparent abnormalities in the operative baseline and help distinguish between preexisting pathology and a technical issue.
The size of the ear canal and whether it is occluded with earwax should also be determined prior to surgery. The presence of wax in the ear canal results in a conductive hearing deficit and will impact the monitoring data. If determined during the preoperative visit, the patient can be asked to clean their ears prior to surgery. If not discovered until the patient is seen in holding, then an ENT consult should be considered for wax removal prior to surgery.
The anesthetic regiment has little to no effect on the potential, and so any concerns or discussions with the anesthesia members of the team are more likely to focus on modalities other than the BAEP [8].
In the operating room, stimulation is usually provided through ear inserts, placed into the ear canal and connected to the electromechanical stimulator through relatively rigid tubing of a known length. The neuromonitorist in the operating room must therefore determine an acceptable location to place the stimulators that will allow them to move with the patient but out of the way of the surgeons. In practice I find affixing them to the Mayfield clamp the most reliable way of performing this important step of setup. Replacing the ear inserts if they fall out during a case can be difficult and is not going to win you much appreciation from the rest of the team.
Stimulation
Clicks
Like any evoked potential, the BAEP is a time-locked (and averaged) response to a given stimulus. In this case, the stimulus is a broadband click, generated by an electromechanical transducer. In the outpatient setting, the stimuli are pure tones of known frequency and are usually generated in the ear pieces of headphones. The objective of the clinical BAEP is to diagnose specific hearing deficits, while in the OR, the objective is to preserve gross hearing. This is the reason broadband clicks are used over pure tones in the OR. Since the large headphones are impractical in the operating room, they are replaced with small transducers and the click is delivered to foam ear inserts through stiff rubber tubing. The length of the tubing is known and therefore imparts a fixed and known delay between the electrical pulse that generates the click, which triggers the recording system, and the delivery of the click to the ear [15]. In most cases, this is a 1 ms delay. The tubing is stiff to allow for reliable delivery of the stimulus to the ear. Care must be taken that the tubing is not pierced which will reduce the amplitude of the delivered click or clamped or kinked which may prevent delivery of the click altogether [16]. It is always worth checking this tubing after positioning of the patient but before the drapes are placed. Once the inserts are placed, sealing the ear canal with bone wax and placing Tegaderm over the ear will prevent fluid from entering.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








