25 Bypass Surgery for Complex MCA Aneurysms
Introduction
Complex MCA aneurysms may be categorized into three major groups based on etiology and anatomic location along the vessel: (1) M1 segment fusiform aneurysms, (2) giant MCA bifurcation aneurysms, and (3) distal MCA infectious aneurysms. Although fusiform aneurysms are relatively uncommon, the M1 segment tends to be the region of the MCA affected, perhaps due to its relationship to atherosclerotic disease.1 Among MCA aneurysms overall, the most common anatomic location is at the MCA bifurcation (or trifurcation) at the origin of the M2 branches, with saccular morphology and etiology likely related to hemodynamic forces. Distal MCA branches such as M2-M4 segments are a common site for infectious or mycotic aneurysms, often associated with favored flow routes of septic emboli from infective endocarditis.
The role of bypass surgery for complex MCA aneurysms has been somewhat tempered by the infrequency of this technically demanding procedure usually done by specialized vascular neurosurgeons at institutions with an ample referral base2 and by the rapid evolution of potential alternative endovascular therapies. Yet bypass is an essential tool in the repertoire of a vascular neurosurgeon given its sometimes crucial role in the management of complex intracranial aneurysms. In this chapter, we focus on surgical bypass treatment options for complex MCA aneurysms, including the decision-making process and details of surgical technique.
Treatment decisions
Although many of the following steps occur in parallel, generally the sequence progresses from the history/exam to imaging, then determination is made as to optimal management options that may include observation, surgical treatments, endovascular treatments, or combined surgical-endovascular treatments. For initial imaging assessment, we use CTA with maximal intensity projections and 3D image reconstruction since it is quick, noninvasive, surveys other related neuroanatomic issues (such as mass effect for giant aneurysms or any associated hydrocephalus), identifies other coexisting aneurysms, assesses the full extent of giant or complex aneurysms (such as thrombosed components) that may not be seen on conventional angiography, and outlines relationships of the target with other anatomic structures such as the skull base or proximity to potential extracranial bypass vessels.3–5 In complex scenarios, the CTA can easily be extended to the neck and aortic arch to help facilitate assessment for endovascular access or an array of surgical options. Bilateral cervical carotid and vertebral systems may also be assessed for outlining the individual’s overall intracranial hemodynamic configuration, and ECA branches such as the superficial temporal artery (STA) can be delineated in detail including 3D reconstruction. Linkage may also be done with image-guidance systems (IGS) to facilitate STA harvest or distal MCA bypass if needed. Our second choice study for those patients who cannot undergo CTA is conventional digital subtraction angiography (DSA) that can be done with contrast-minimizing strategies if needed, or least commonly MRA.
Complex MCA aneurysms that cannot be appropriately treated by surgical or endovascular trapping with possible bypass may alternatively be treated by altering blood flow to the inflow zone using various surgical and endovascular strategies.6 Endovascular options for complex MCA aneurysms may include coil or Onyx embolization (with possible stent or balloon assistance) versus flow alteration strategies or combined surgical-endovascular therapies with bypass followed by endovascular trapping or embolization.7,8 Recurrences are another category that may need special consideration and may be treated with additional surgical clipping, endovascular embolization, proximal parent vessel occlusion, or bypass with surgical or endovascular trapping.9 At our institution, complex elective cases are usually discussed in a weekly multi-disciplinary neurovascular conference involving neurosurgeons, neurologists, and interventional radiologists before final therapies are pursued. As previously mentioned, we will focus on three types of complex MCA aneurysms, which include fusiform M1 segment aneurysms, large or giant MCA bifurcation aneurysms, and distal MCA infectious aneurysms.
M1 fusiform aneurysms
Patients with M1 fusiform aneurysms may present with ischemic symptoms, stroke, mass effect, rupture with subarachnoid hemorrhage, or as an incidental finding. For a focal M1 segment fusiform aneurysm, treatment options include observation with expectant management, endovascular reconstruction, surgical trapping with EC-IC bypass, or combined endovascular-surgical therapies.10 The etiology may involve acute dissection associated with trauma versus chronic fusiform aneurysm, with the natural history of the latter being poorly understood.11 Acute dissecting aneurysms can have significant risk of associated hemorrhage.12 For chronic fusiform aneurysms, cerebral infarction related to thrombus involving compromise of perforators or thromboembolic phenomena may occur more frequently than hemorrhagic rupture based on the natural history of the more frequently encountered vertebrobasilar dolichoectasia or fusiform aneurysms.13 Treatment of choice may depend on whether the aneurysm is ruptured, unruptured but symptomatic, flow-limiting, or incidental upon presentation.
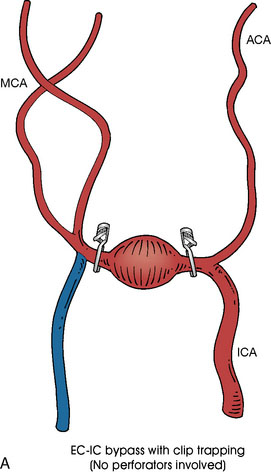
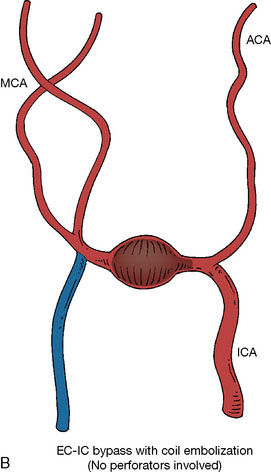
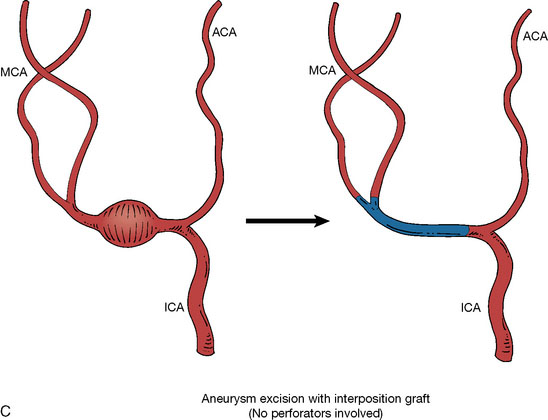
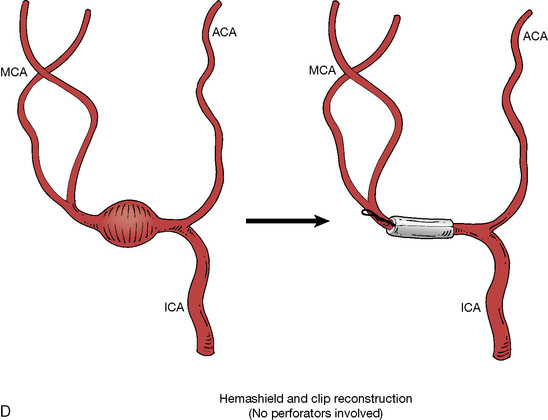
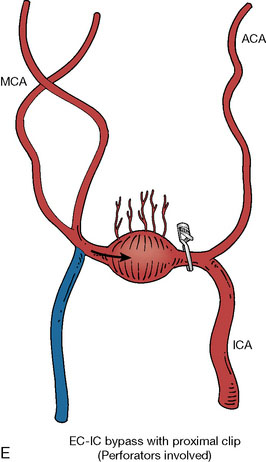
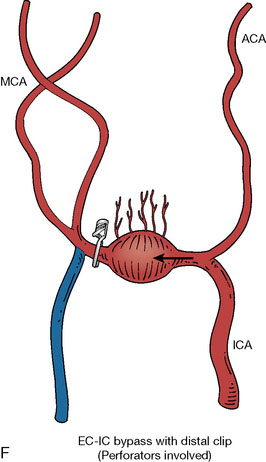
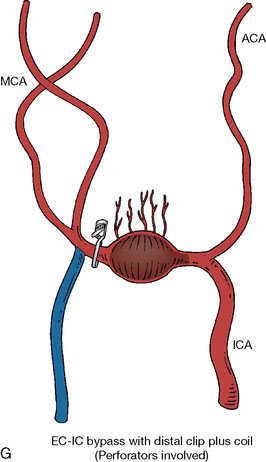
In addition, an important factor for determining appropriate treatment to minimize morbidity is the identification of significant perforators, which may emanate from the aneurysm itself and may not be fully recognized until intraoperatively. If perforators are not involved with the aneurysm, then the treatment goal is exclusion of the aneurysm from the circulation while preserving distal MCA flow using techniques such as EC-IC bypass with clip trapping or coil embolization (Figures 25–1A and 25–1B), with less favored alternatives being excision of aneurysm with interposition graft or aneurysmal vessel reconstruction depending on the aneurysm configuration (Figures 25–1C and 25–1D). If perforators are involved, then flow remodeling strategies to reduce hemodynamic pressures at the inflow zone may be pursued such as EC-IC bypass with proximal clip (retrograde flow supplying perforators), and less favorably EC-IC bypass with distal clip, which may be supplemented with additional endovascular embolization as needed (Figures 25–1E through 25–1G). Intraoperative angiography can be helpful in determining the optimal configuration of flow remodeling. Various techniques for IC-IC bypass with aneurysm trapping have also been described.14,15 At least the following additional factors are also considered for therapeutic selection: overall medical condition and age of the patient along with anatomic extent of the fusiform segment. Diffuse extent of fusiform aneurysms may preclude a focused surgical therapy and warrant conservative management with observation and possibly antiplatelet or anticoagulation agents to reduce risk of thromboembolic phenomena related to turbulent flow (Figures 25–2A through 25–2C).
Acute traumatic arterial dissection involving a focal M1 segment is rare, but if the resulting fusiform aneurysm with dissected flap demonstrates significant flow limitation, associated pseudoaneurysm, or rupture in a patient whose overall medical condition would tolerate invasive therapies, options include surgical trapping with bypass, endovascular takedown with bypass, or endovascular reconstruction. EC-IC bypass followed by proximal surgical occlusion alone without distal trapping may result in reversal of blood flow in the M1 segment with preserved flow to the M1 perforators while helping the dissection subside due to the direction of orientation of the dissected flap.16 Surgical trapping may alternatively be accomplished in conjunction with a purely intracranial IC-IC bypass.14,15 Endovascular takedown with EC-IC bypass may be a more attractive option if the fusiform segment involves the distal internal carotid artery near the skull base in addition to the M1 segment (with ipsilateral anterior cerebral artery having potential contralateral collateral supply via the anterior communicating artery), but surgical therapy may still be achieved in this scenario.17 Reconstructive endovascular options may include flow diverting stent placement, self-expandable stent-within-stent placement, and stent with balloon-assisted coiling.18
Giant MCA bifurcation aneurysms
Patients with giant MCA bifurcation aneurysms may present with mass effect, seizures, ischemic symptoms, stroke, rupture with subarachnoid hemorrhage, or as an incidental finding. For giant MCA bifurcation aneurysms of saccular or fusiform morphology, the configuration of the bifurcation or trifurcation on imaging should help determine how to best preserve the M2 branches. The surgical option of simply clipping the neck of the aneurysm and preserving the direct flow from the M1 trunk to the M2 branches may be precluded by calcified atheromatous base, thick walls with giant wide neck, extensive thrombosis, or anatomic configuration of the M2 origins. Booster clips or a complex combination of clips may be required to achieve clipping of giant aneurysms, and the use of a vascular clamp for assistance has also been described.19 Alternatively, surgical or endovascular obliteration may be performed with or without bypass.20,21
If the anatomic configuration is amenable, EC-IC bypass may be performed to one nonsalvageable M2 branch and clipping can then be performed across the aneurysm neck along with this revascularized M2 origin, while flow may be preserved from the M1 segment to the other salvageable M2 trunk.22 If the giant MCA bifurcation aneurysm is to be excised instead of clipped, the bifurcation can still be microsurgically reconstructed with similar methods.23,24 An analogous reconstruction option can include excision of the giant MCA bifurcation aneurysm with end-to-end anastomosis of the remaining M1 segment to one M2 trunk and performing STA-MCA bypass to the other M2 trunk.25 Sometimes EC-IC or IC-IC bypass may be done to a dominant inferior (or superior) segment if the other bifurcation branch provides minimal supply or has already suffered significant previous infarct with minimal preservable cerebral tissue to salvage in that region. For large or giant MCA aneurysms distal to the bifurcation, surgical trapping may similarly be performed with possible EC-IC bypass or end-to-end anastomosis.26