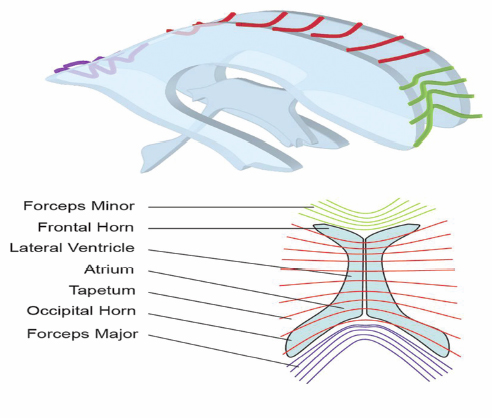
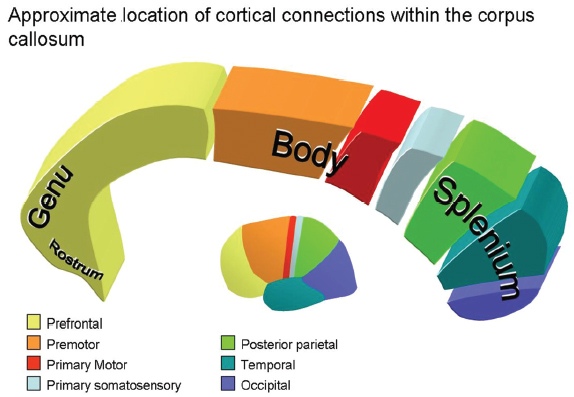
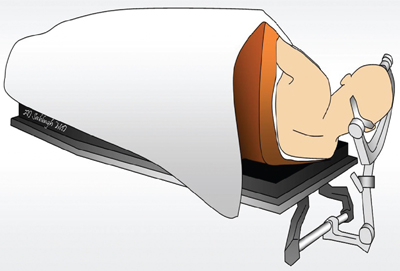
13 Callosotomy The goal of the majority of surgical and medical therapies for the treatment of epilepsy is the complete cessation of seizures with the preservation of maximal brain function. Despite this, there remain syndromes where curative procedures are not a relevant consideration and palliative approaches toward the control of symptoms or the reduction of seizure burden, with associated gains in the quality of life (QOL), are more appropriate goals. Increasing variety in the pharmacotherapy of epilepsy has reduced the role surgery plays in such cases. Division of the corpus callosum to limit the spread of ictal discharges and thus reduce generalized epileptic symptomatology remains a procedure of some utility. Newer palliative options such as vagal nerve stimulation (VNS) have attempted to provide a similar QOL gain with reductions in the potential neurological morbidity associated with the procedure. Nevertheless, in carefully selected patients callosotomy remains an effective tool to control atonic seizures specifically, with an acceptable incidence of significant neurological morbidity. Surgical sectioning of the corpus callosum was first performed by Dandy, to access the third ventricle.1 In 1940, Van Wagenen and Herren proposed that division of commissural pathways in the corpus callosum might be helpful to treat epilepsy. 2 They stated that this surgery “may serve to limit the spread of an epileptic wave to the opposite hemisphere.” Several observations in patients with long-standing epilepsy that improved or resolved after neoplastic, ischemic, or traumatic damage to corpus callosum led them to propose this procedure. They described partial callosotomy from the genu and the body of the corpus callosum up to and/or including the splenium, in combination with sectioning the fornix. Eight out of 10 patients who underwent the procedure stopped having convulsive seizures impairing consciousness.2 A decade later Kopeloff et al experimented with callosotomy on monkeys.3 The surgery was reintroduced in the 1960s, by Bogen and colleagues. Their experience was detailed in several consecutive reports during the 1970s.4–7 The procedure gained acceptance quickly and several institutes in the ’80s and ’90s reported on a large number of corpus callosotomies, including the Montreal Neurological Institute,8,9 Yale University,10–13 and Dartmouth-Hitchcock Medical Centre in New Hampshire,14 with promising results. The advent of VNS, a less invasive procedure with similar indications (i.e., relief of atonic seizures), led to a reduction in callosotomy frequency in recent years. Incomplete seizure control following VNS insertion has led to a redefinition of the role of callosotomy in the management of refractory seizures. The corpus callosum is the largest identifiable white matter fiber bundle in the brain. It interconnects the hemispheres, mostly contralateral homotopic structures, but there are also connections between heterotopic areas of brain. It contains more than 3 x 108 fibers.15 The mean measurements of the callosum on magnetic resonance imaging (MRI) and cadaver dissection are as follows: length, 74.9 mm and 87.2 mm; maximum height, 25.5 mm and 19.7 mm, respectively.16 It is generally agreed upon that the corpus callosum, at its midsagittal plane, is comprised of five anatomical segments: the rostrum, genu, body, isthmus, and splenium. Forceps minor and major are large callosal fiber tracts arising from the genu and the splenium, respectively (Fig. 13.1). They are best identified in the axial plane. Several authors further classify the midsagittal plane regions of the corpus callosum from geometric, topographic, and morphometric perspectives. Witelson17 subdivided the corpus callosum geometrically into (1) the anterior third (region I containing fibers projecting into prefrontal, premotor, and supplementary motor regions); (2) the anterior midbody (region II with callosal motor fiber bundles); (3) the posterior midbody (region III with somaesthetic, posterior parietal projections); (4) the isthmus (region IV with posterior parietal, superior temporal projections); and (5) the splenium (region V with occipital, inferior temporal projections). Recently, diffusion tensor magnetic resonance imaging (dtMRI) fiber tractography has been used by Hofer and Frahm15 to outline five regional partitions of the corpus callosum in live human subjects. Region I, the anterior sixth, includes fibers going to the prefrontal region. Region II, the rest of the anterior half, contains fibers projecting to pre-motor and supplementary motor cortical areas. This would be the largest subdivision of the corpus callosum. Region III is the posterior half without the posterior third, and it contains fibers crossing to the primary motor cortex (unlike the Witelson classification). Region IV is the posterior one-third minus the posterior one-fourth. It contains primary sensory fibers. Region V, is the posterior one-fourth, and its fibers cross to parietal, temporal, and occipital areas (Fig. 13.2). Fig. 13.1 Major pathways of corpus callosum in relation to periventricular anatomy. The corpus callosum represents the largest component of the ventricular lining.18 The rostrum forms the floor of the frontal horn. Forceps minor forms the anterior wall of the frontal horn. The genu and body of the corpus callosum form the roof of the frontal horn and the body of the lateral ventricle. Forceps major produces a prominence called the bulb in the upper part of the medial wall of the atrium and occipital horn. Another fiber tract, the tapetum, which arises in the posterior part of the body and splenium, sweeps laterally and inferiorly to form the roof and lateral wall of the atrium and the temporal and occipital horns18 (Fig. 13.1). The arterial supply to the corpus callosum delivers blood from the outer surface in, whereas the venous drainage forms in the inner ependymal surface19 and drains in the deep venous system. Three main arteries contribute to the arterial supply of the corpus callosum, the anterior communicating artery, the pericallosal artery, and the posterior pericallosal artery. The anterior communicating artery contributes branches; the subcallosal and the median callosal arteries.20 The former is present in 50% of cases and is a single dominant artery that supplies the rostrum and genu of the corpus callosum. It also supplies the anterior hypothalamus, the subcallosal area, the fornix, and the medial portion of the anterior commissure.20 The median callosal artery is present in 30% of cases and may contribute to the blood supply of the body, and occasionally the splenium.20 Fig. 13.2 Connecting fibers of major brain regions and their anatomical relationships within the corpus callosum. The pericallosal artery runs in the callosal sulcus in ~60% of cases.20 It branches into the short and long callosal arteries.21 The short callosal arteries are present in 98% of hemispheres; they directly penetrate the corpus callosum and provide it with significant arterial supply.21 There are around seven such arteries on either side. The long callosal arteries, on the other hand, are fewer in number and are less commonly present.20,21 They contribute to the formation of the pericallosal pial plexus and provide a source of anastomoses with the posterior callosal artery.20 Ture et al further subclassified the short arteries into the callosal, cingulocallosal, and long callosal arteries that form the pericallosal pial plexus and the recurrent cingulocallosal artery.20 The posterior pericallosal artery is present two-thirds of the time.22 After branching off the posterior cerebral artery, at the level of the splenium, it splits into superior and inferior branches at this level.20 The superior branch anastomoses with branches from the pericallosal artery. The anastomoses between the pericallosal artery and posterior callosal artery occur at the splenium in the majority of cases.20,22–24 The posterior cerebral artery also indirectly supplies the corpus callosum through choroidal branches.19 Callosal and calloso-cingulate (also called transcallosal) veins drain blood from the corpus callosum and course in the direction of the lateral ventricles, to drain into the septal and atrial veins. The septal and atrial veins drain into the deep central cerebral veins.19 Prior to proposing corpus callosotomy for the control of refractory seizures in a given patient, several admissibility criteria must be met. First, the patient must clearly demonstrate medical intractability.25 Several authors have attempted to define failure to antiepileptic medication.25–31 Most agree that epilepsy spanning 18 months, failing two antiepileptic medications with an average seizure frequency of one seizure per month, and no period of freedom of seizures longer than 2 months is considered intractable epilepsy.27 Given that callosotomy is a palliative treatment aimed at seizure reduction rather than seizure cure, the team considering the patient for seizure surgery should ensure that there is no clearly defined focal onset for the seizure disorder based on imaging and electrographic data including single photon emission tomography (SPECT) scans, both ictal and interictal modes, as well as detailed MRI, and video electroencephalogram (EEG) telemetry. In children, in particular, even infantile spasms can occasionally be related to a focal anatomical or physiological anomaly of the brain.32–34 In these cases resective surgery should be offered to a patient because of the higher incidence of cure of seizures, the ultimate “focal” resection being a complete hemispherectomy. In cases where anatomical or electrographic damage appears to be clearly bilateral, in patients where the bilateral disorders are primarily frontal, and in patients who exhibit developmental difficulties as well as multiple seizure types with bilateral but initially focalized onset, callosotomy can be considered. The primary seizure type that responds to callosotomy is the atonic seizure, whereas repeated generalized tonic, tonic clonic, and myoclonic seizures tend to respond less well.35–39 The chance of the procedure exhibiting a significant impact on the QOL of these patients is proportional to the extent of the disconnection.36 Very severely involved children and adults with Lennox-Gastaut syndrome, in particular, are very unlikely to suffer from disconnection syndrome following an extensive callosotomy because of their baseline poor neurological development. It becomes clear in those patients that a more extensive callosotomy, preferably in one stage, is a good therapeutic option. The advent of vagus nerve stimulation for the palliation of severe seizures disorders has put in question the traditional indications for callosotomy. Vagus nerve stimulation, although associated with less immediate morbidity (except, perhaps, in patients with pseudobulbar states) remains a “technology driven” treatment that is, therefore, expensive over a lifetime to maintain, and is not readily accessible in all areas of the globe. Given that it is less invasive than callosotomy, it is, however, likely to be the first line of treatment for patients with multiple seizure types, poor development, and no evidence of unilateral focality for resective surgery. We have had the opportunity of offering callosotomy to two patients who had had prior VNS implantations but were still manifesting frequent atonic seizures. In both patients, an anterior two-thirds callosotomy was successful at controlling the atonic seizures after the VNS had led to a reduction of other seizure types, including generalized epilepsy. In most refractory epilepsy cases, with significant atonic seizures, callosotomy is performed over the anterior one-half to two-thirds. More rarely, for seizure spread in posterior brain areas, exclusively posterior sectioning is performed. Complete sectioning of the corpus has been performed as a multistaged operation, but some authors advocate performing callosotomy up to the splenium in a single position and single surgery.39 Others have been able to completely section the corpus callosum including the splenium with one procedure.35 This may be particularly useful in severe Lennox-Gastaut cases. Neuronavigation has been very helpful in confirming intraoperatively that the desired extent of callosal section has been attained. Patients undergoing standard anterior corpus callosotomy will usually be positioned supine using head fixation, and appropriate padding for the body and extremities. Patient positioning for anterior callosotomy is most often performed such that the body of the corpus callosum is perpendicular to the floor, that is, with the head in a neutral position and with a 20-degree flexion of the head.40 Some have advocated that the head be rotated toward the floor, either to have the falx parallel with the floor, or to a somewhat more limited extent, such that the frontal lobe on the dependent side would fall away from the falx with gravity rather than retraction (Figs. 13.3 and 13.4). On the other hand, the surgeon’s orientation may be less “natural” in this position than if the head is placed in a neutral orientation. Neuronavigation systems can be quite helpful in the planning of the surgery, both to locate the actual craniotomy and to determine the side of the craniotomy, away from the predominance of cortical draining veins.41
History
Anatomy
Topographic Anatomy
Surgical and Vascular Anatomy
Indications
Approaches
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








