Patient characteristics
Treatment-related factors
Cancer-related factors
Older age
Prolonged immobility
Prior history of thrombosis
Elevated leukocyte and platelet counts
Acute infection
Comorbidity
Obesity
Chemotherapy
Hormonal agents
Growth factors
Antiangiogenic agents
Major surgery
Central venous catheters
Primary site of cancer
Stage of cancer
Compression of directly invasion of large vessels
Mucin from adenocarcinoma
Tissue factor expression
In regard to cancer-related factors, malignant brain tumors, hematological malignancies, and adenocarcinomas of the pancreas, stomach, ovary, uterus, lungs, and kidneys were related to highest risk for development of venous thromboembolism (VTE) through large epidemiological studies [12]. Moreover, advanced stage or metastatic cancer has been shown to be associated with an increased risk of VTE compared with early-stage or localized cancers. Besides, direct compression or invasion of large blood vessels due to the tumor itself is one of the important causes of cancer-associated thrombosis.
Malignant cells themselves activate the coagulation cascade through multiple mechanisms, i.e., inflammatory cytokines, tissue factor (TF) expression, and procoagulant expression by cancer cells. When monocytes or macrophages interact with cancer cells, they release tumor necrosis factor, interleukin-1, and interleukin-6, causing endothelial damage and disruption (Fig. 19.1). Moreover, cancer cells activate monocytes or macrophages to release TF. TF directly induces the conversion of factor VII to factor VIIa, leading to the subsequent activation of the extrinsic pathway of the coagulation cascade, which brings out thrombin generation and thrombosis. Procoagulants are expressed by cancer cells and normal cells. It directly accelerates the conversion of factor X to factor Xa. In one side, advanced stage cancer cells often express large amount of sialic acid-rich glycoproteins. The sialic acid in mucin from adenocarcinomas can cause a direct nonenzymatic activation of factor X [13]. To prevent cancer cell-related thrombosis through various mechanisms, unfractionated heparin is considered much more effective than warfarin.
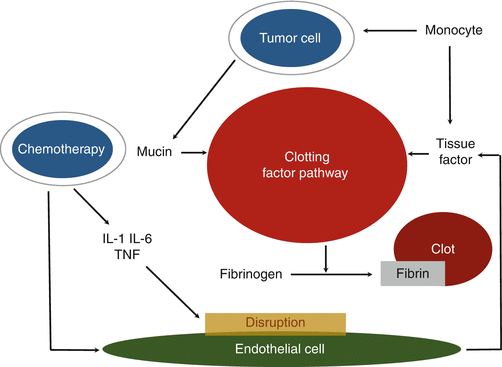

Fig. 19.1
Multiple mechanisms in Trousseau’s syndrome. When cells of the monocyte or macrophage interact with malignant cells, they release tumor necrosis factor, interleukin-1, and interleukin-6, causing endothelial damage and disruption. Monocytes or macrophages are also induced by cancer cells to release tissue factor. Tissue factor directly induces activation of clotting factor pathway resulting in thrombosis. The interaction between tumor cells and macrophages also activates platelets and clotting factor pathway, which leads to the generation of thrombin and thrombosis. The mucin and procoagulants produced from adenocarcinomas cause activation of clotting factor pathway. In addition, aggressive chemotherapy itself increases the risk of thrombosis
19.4 Management of Cancer-Related Stroke
Regardless of underlying mechanisms, the primary approach to treating TS is to eliminate the causative tumor as soon as possible. Treatment and prevention of cancer-associated thrombosis should focus on reducing mortality and morbidity and improving the quality of life in patients with malignancy. Furthermore, benefits of anticoagulation therapy should always surpass the risk of bleeding because the bleeding is the most harmful or devastating effect of anticoagulant use.
For a long time, warfarin has been the standard therapy for chronic anticoagulation but in the cancer population is highly associated with bleeding complication, recurrent venous thromboembolism, and drug–nutrient and drug–drug interactions. Low-molecular-weight heparin (LMWH) is generally preferred to unfractionated heparin because LMWH does not need frequent monitoring with blood tests. Several randomized controlled trials compared the efficacy and safety of LMWH with warfarin for the treatment of VTE. The Comparison of Low-Molecular-Weight Heparin versus Oral Anticoagulant Therapy (CLOT) study published in 2003 was the largest randomized controlled multicenter trial [14]. This study enrolled 672 patients (336 patients in the dalteparin group and 336 patients in the oral-anticoagulant group) with cancer and acute VTE, and it compared 6 months of treatment with warfarin and the LMWH dalteparin at a dose of 200 IU/kg body weight, administered once daily subcutaneously. The probability of recurrent thromboembolism at 6 months was 17% in the warfarin group and 9% in the dalteparin group. The hazard ratio for recurrent thromboembolism in the dalteparin group as compared with the oral-anticoagulant group was 0.48 over the 6-month study period, which was statistically significant [14]. No significant differences between the dalteparin and warfarin groups were detected in the rate of bleeding (14 and 19%, respectively) and the mortality rate at 6 months (39 and 41%, respectively). Based on this result, LMWH is recommended for both the initial and maintenance therapies of cancer-associated thrombosis by major international guidelines (Table 19.2) [15, 16].
Table 19.2
Guidelines on treatment of venous thromboembolism in patients with cancer
NCCN 2014 | ASCO 2014
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|
|---|





