Cardiovascular disease
Odds ratio (confidence interval 95 %)
Arterial hypertension
1.37 (1.03–1.83)
Heart failure
2.38 (1.22–4.62)
Ischemic heart failure
1.27 (0.99–1.62)
Ischemic stroke
1.58 (1.02–2.46)
Atrial fibrillation
4.02 (1.03–15.5)
Pulmonary hypertension
1.4 (1.1–2.8)
General mortality
6.24 (2.01–19.4)
A large cohort study in Spain also investigated cardiovascular risk and outcome in patients with sleep-related breathing disorders. The study compared snoring, different severities of sleep apnea, and treated versus untreated patients with sleep-disordered breathing [17]. An increased mortality and an increased risk for cardiovascular incidents in patients with sleep apnea depending on severity were shown.
Patients with an apnea–hypopnea index (AHI) above 30 events per hour have an approximately twofold higher risk for fatal and nonfatal cardiovascular events as well as for developing arterial hypertension [17]. Patients with a regular use of CPAP therapy had a comparable mortality and cardiovascular outcome as in healthy subjects.
New population-based cohort studies including sleep investigations can clarify if sleep-related cardiovascular disorders and sleep-disordered breathing are linked or independent of each other. These new studies are currently ongoing. The Framingham study plans to add a sleep module. The study of health in Pomerania, a region in northern Germany with a high prevalence of arterial hypertension did add a sleep module with cardiorespiratory polysomnography for one night; 1269 out of more than 6700 randomly selected participants underwent a night in the sleep laboratory [18].
Sleep and the Autonomous Nervous System
From physiological investigations, we know that during non-REM and REM sleep autonomic controls are different [19]. During non-REM sleep, sympathetic activity decreases and is lowest during slow-wave sleep. In contrast, the vagal component is highest during slow-wave sleep with a decreased heart rate and blood pressure under normal conditions. During REM sleep, sympathetic activity is variable with intermittent high surges of activity followed by cessations. The average sympathetic activity recorded during REM sleep by neurography on the peroneal nerve is similar to that during wakefulness but higher than during non-REM sleep. Vagal activity during REM sleep is not as high as during slow-wave sleep. For vagal activity, only indirect parameters (e.g., derived from heart rate variability) are taken and no direct continuous variable was assessed so far.
Sleep-disordered breathing is accompanied by cyclical variation of heart rate (Fig. 47.1). This cyclical variation of heart rate had been described very early, shortly after the description of sleep apnea [20]. This cyclic heart rate variation is so typical, that it can be used to diagnose sleep apnea from the heart rate pattern alone [10]. Combining the results of previous studies, it is even possible to distinguish sleep stages and the severity of sleep apnea to some degree [21].
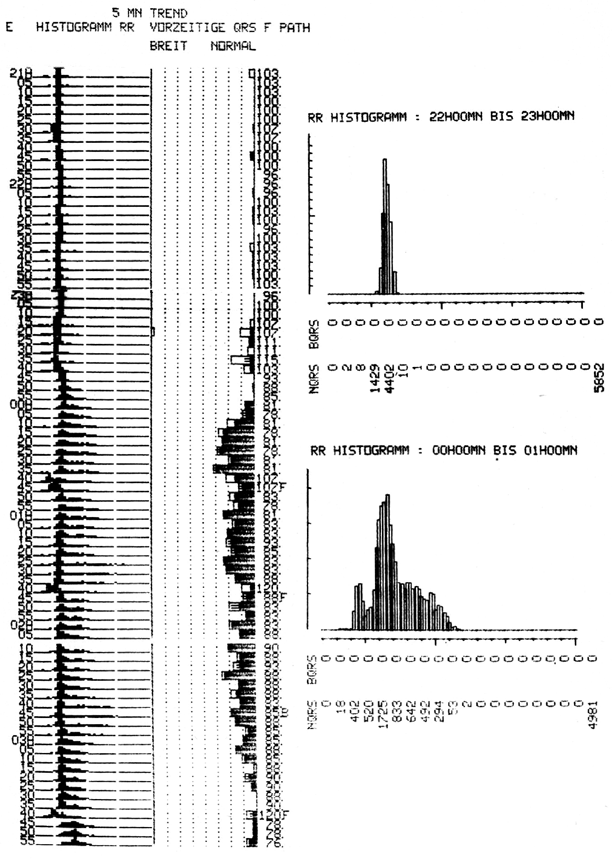

Fig. 47.1
Holter ECG recording of a patient with sleep apnea and an extremely high variability of heart rate during the night. The ECG recording and the tape analysis were performed in spring 1982. At the time of recording, it was common to visualize 1-h distributions of RR intervals. The comparison of the two 1-h histograms shows an episode of regular sleep with low variability (22–23 h) and an episode with very high variability just 2 h later (0–1 h). ECG electrocardiogram
The autonomous nervous system also acts on the vascular tone during sleep. During REM sleep with a higher activity of sympathetic tone, a higher vascular tone resulting in a higher blood pressure is the consequence. If blood pressure is recorded with a sufficiently high resolution in amplitude and time, REM sleep can be identified in healthy subjects. Besides blood pressure, a couple of years ago a new technique was introduced to record vascular tone noninvasively and continuously. This is called peripheral arterial tonometry (PAT) [22]. Many studies had been conducted and the signal had proven to be a very useful marker in the recognition of sleep-disordered breathing through the autonomic responses [23]. Based on arterial tone, it is possible to detect arousals and differentiation between non-REM sleep and REM sleep [24].
Sleep and the Heart
Heart rate is regarded as a direct mirror to sympathetic activity. A decrease in heart rate during slow-wave sleep and a higher heart rate variability during REM sleep reflects up- and downregulation of sympathetic activity and corresponds to arrhythmias observed during sleep. Rhythm-related problems during sleep can be bradycardia, heart block of different types, and atrial fibrillation. Most often, arrhythmias are related to other underlying cardiac disorders and they have their specific expression during sleep as well. Some arrhythmias are related to specific sleep stages. During sleep-disordered breathing, one can observe bradycardia, other low-frequency arrhythmias, or even heart block at the end of apneic events. An impressive example of sleep-related arrhythmia of this type is depicted in Fig. 47.2. No direct or causal relationship amongst arrhythmias, sleep stages, and apneic events has been shown statistically, because these arrhythmias are rare events.
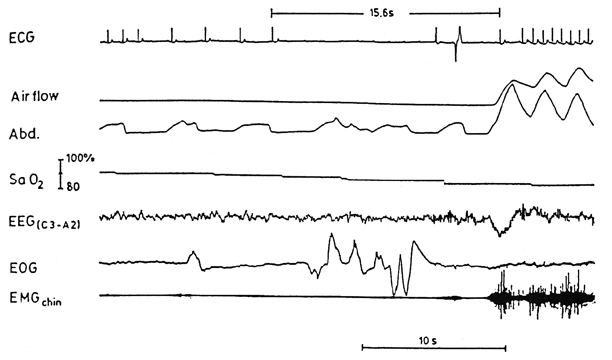

Fig. 47.2
The ECG recording in the sleep laboratory during the 1980s shows a cardiac arrest with 15.6 s within one supraventricular beat and one ectopic beat observed. This was observed at the end of an obstructive apnea event and during REM sleep. ECG electrocardiogram, REM rapid eye movement
Heart failure has a high prevalence in persons above the age of 45 in Western countries [25]. Systolic and diastolic left ventricular dysfunctions are reported with prevalence between 5 and 20 %. Mortality of heart failure is high. Sleep stages and altered regulation have a strong impact on heart function during the night. This is important in patients with heart failure because sleep apnea can trigger a reduction in the ejection fraction of the left ventricle.
The prevalence of sleep-disordered breathing is very high in patients with heart failure [26]. Studies report between 30 and 50 % of the patients suffering from Cheyne–Stokes breathing and central sleep apnea. The Cheyne–Stokes breathing is characterized by a waxing and waning of respiratory amplitude which may assume very low amplitudes resembling just central apnea events. Another 20–40 % suffer from obstructive sleep apnea [27]. There are several potential mechanisms which link heart failure and sleep-disordered breathing. Heart failure, whether systolic or diastolic, increases the risk for central respiratory events, creating a vicious cycle between sleep apnea and heart failure. Whether treatment of apnea with CPAP lowers mortality in these patients is not finally clarified. A large study did not show a beneficial effect regarding mortality [28]. A post hoc sub-study analysis did show, however, a beneficial effect on patients in lowering central apnea in heart failure and increasing left ventricular ejection fraction [29]. Further investigation is needed to identify subpopulations who might benefit from treatment with CPAP .
Ischemic heart disease appears to be increased in patients with sleep-disordered breathing [30]. Hypoxemia during apnea events presents the pathophysiological mechanism of this complication. It is not clarified whether hypoxemia leads to a cumulative or an acute effect on the coronary vessels. It is even thought that re-oxygenation after each single apnea event might present an even more severe stress to the vessels. However, this association is not fully explored, neither by epidemiological studies nor by pathophysiological studies.
Sleep and the Vascular System
The association which has been found most consistently over a large number of studies is the association between sleep-disordered breathing and hypertension. [31–33]. In various well-designed placebo-controlled studies, the treatment of obstructive sleep apneas could show a modest but significant reduction in diurnal and nocturnal systolic and diastolic blood pressure [34, 35]. Meta-analyses on treatment studies also did show a great variety of effects on blood pressure, and therefore subgroups of sleep apnea patients who might benefit most have to be defined. Therapy-resistant arterial hypertension showed a significant reduction after treatment and this is important as more than 90 % of men and 60 % of women with a resistant hypertension have a sleep apnea syndrome [36]. In this group, CPAP seems to be more effective in lowering systolic blood pressure by 15 mmHg and diastolic blood pressure by 8 mmHg. Knowing that a reduction in systolic blood pressure by 10 mmHg lowers the risk of coronary artery disease by 22 % and risk of stroke by 41 %, physicians, cardiologists particularly should be aware of this relationship. A recognition of this led to the adoption of guidelines for the management of hypertension [3]. Besides this clear evidence, there are a number of other factors which are worth noting. Blood pressure increases during prolonged episodes of apnea leading to dangerous situations with very high blood pressure peaks (Fig. 47.3). There seem to be cumulative effects of successive apnea and hypopnea events which can cause hypertensive crisis at the end. This may be a potential mechanism for sudden cardiac death in these patients. Another mechanism is the role of non-REM and REM sleep transitions with a sudden rise in blood pressure. This rise in blood pressure is observed in both systolic and diastolic values (Fig. 47.4). The rise of blood pressure is so clearly linked to the onset of REM sleep that this can be only explained by an immediate change of vascular resistance with the changed level of sympathetic activity. This supports the finding that PAT, which just reflects vascular tone, can be used very reliably to detect REM sleep. It would be challenging to investigate in how far this effect of activation is lost in patients with autonomic neuropathy or in patients with diabetes with blunted autonomic nervous system activity. Since we know that sleep apnea impairs autonomic responses, one might expect that this non-REM/REM transition response is impaired. But in general this does not seem to be the case. Possibly different degrees of disease progression might be distinguished by testing this change in autonomic nervous system response to sleep stage changes.
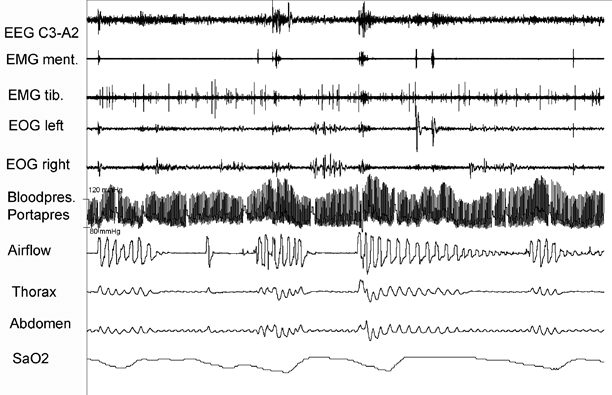

Fig. 47.3
The recording shows a 5-min period with very high blood pressure peaks during REM sleep at the end of apnea events. The patient was a 70-year-old woman with treated hypertension, obstructive sleep apnea, and restless legs syndrome. The episodes of apnea are accompanied by excessive blood pressure peaks