Chapter 11 Carotid stenting (CS) has evolved to become a widely utilized method of carotid artery revascularization. Improved periprocedural outcomes have been achieved through technical and procedural innovations including improved devices, increased operator experience, and a better understanding of optimal technique, as well as the importance of patient selection. Results of several randomized trials showed that carotid endarterectomy (CEA) was more effective than medical therapy in reducing the risk of stroke.1–5 Thus, the American Heart Association (AHA) guidelines recommend CEA for symptomatic carotid stenosis >50%, and for asymptomatic carotid stenosis >60% if these asymptomatic patients also have an expected life expectancy of 5 years, provided the periprocedural complication rates are less than 6% and 3%, respectively.6,7 During the late 1980s, several investigators began to apply the percutaneous approach to carotid revascularization.8,9 Early results by Roubin and others10,11 showed that CS was feasible and had acceptable complication rates despite the novel experience with this technique, use of primitive equipment, and lack of distal embolic protection devices (EPDs). Subsequently, long-term (up to 5 years) follow-up demonstrated that CS could be accomplished with acceptable complication rates and with durable results.12 Several studies have demonstrated equivalence of CS versus CEA. The first was the Carotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS), randomizing mostly symptomatic patients (90%) who were at standard surgical risk. The 30-day stroke or death rate (10% CS vs 9.9% CEA) and 3-year ipsilateral stroke rate were not different.13 The Stenting and Angioplasty with Protection in Patients at High Risk for Endarterectomy (SAPPHIRE) Trial14 – a multicenter randomized trial of high surgical risk patients with either symptomatic >50% carotid stenosis or asymptomatic >80% carotid stenosis – demonstrated that CS was noninferior to CEA with myocardial infarction (MI)/stroke/death rates of 12.2% vs 20.1%, respectively, at 1 year, with clinical equipoise maintained at 3 years.15 The Clopidogrel and Aspirin for Reduction of Emboli in Symptomatic Carotid Stenosis (CaRESS) Trial, a multicenter, nonrandomized prospective comparative study of symptomatic and asymptomatic patients at high or low surgical risk, revealed no significant differences in the 30-day and 1-year stroke or death rates between CS and CEA (2.1% vs 3.6% and 10.0% vs 13.6%, respectively).16 Recently, the Endarterectomy versus Angioplasty in Patients with Symptomatic Severe Carotid Stenosis (EVA-3S) and Stent-Protected Angioplasty versus Carotid Endarterectomy (SPACE) Trials randomizing symptomatic patients at normal surgical-risk however, showed that CS did not achieve parity with CEA.17,18 Although 30-day ipsilateral stroke or death rates in the SPACE Trial were similar (CS 6.84% vs CEA 6.34%), CS was not proved noninferior.17 In the EVA-3S study, not only was noninferiority not reached, CS performed worse, with a higher 30-day stroke or death rate (9.6% vs 3.9%, p = 0.01).18 The indications for carotid stenting are similar to those for CEA if the procedure can be accomplished with event rates within the American Heart Association (AHA) guidelines. Patients with increased surgical risk due to comorbidities such as severe cardiac or pulmonary disease or anatomic factors such as high lesions behind the mandible (C2 and above), low lesions (necessitating thoracic exposure), restenosis after previous CEA, contralateral internal carotid artery (ICA) occlusion and prior neck dissection/irradiation may be ideal candidates for CS. With present-day devices and technologies, almost any carotid lesion can be stented. It is not a question, however, of an operator being able to access the lesion or eliminate the stenosis by placing a stent, but is absolutely related to the ability to perform these tasks safely with a low stroke/death rate. Thus, several situations increase the likelihood of adverse events during CS (i.e., relative contraindications): These patients may be more safely treated with CEA or medical therapy. Fig. 11.1 Angiogram demonstrating severe distal tortuosity, increasing the difficulty of placing a distal filter in a “safe landing zone.” Similar to guideline recommendations,19 we advocate that experienced operators study both carotid arteries, with optional angiographic imaging of a vertebral artery if that can be safely performed. One of the most important points to note is that the final decision to proceed with CS should be made after adequate carotid/cerebral angiograms have been performed, and the sheath placed in the CCA. Often, certain anatomic findings (e.g., vascular tortuosity, heavy concentric lesion calcification) that significantly increase stent risk are first detected during angiography, especially after sheath or guide placement. Depending on these findings, the decision to proceed with CS should be carefully reconsidered and the procedure terminated, if appropriate. As experience with CS has accumulated, we have identified clinical and anatomic markers for increased stroke risk during CS (high stent risk).20 These are age, reduced cerebral reserve, excessive vascular tortuosity, and heavy concentric lesion calcification (Table 11.1). Patients with any two or more of the four risk markers should be excluded from CS because the risk of periprocedural stroke will be excessive. Alternative therapies, either medical or surgical, are recommended for these patients.
Carotid Artery Stenting
 Historical Perspective
Historical Perspective
 Multicenter Clinical Trials
Multicenter Clinical Trials
 Indications and Contraindications of Carotid Stenting
Indications and Contraindications of Carotid Stenting
 Inability of the patient to tolerate the dual antiplatelet agents that are mandatory for at least 1 month after CS
Inability of the patient to tolerate the dual antiplatelet agents that are mandatory for at least 1 month after CS
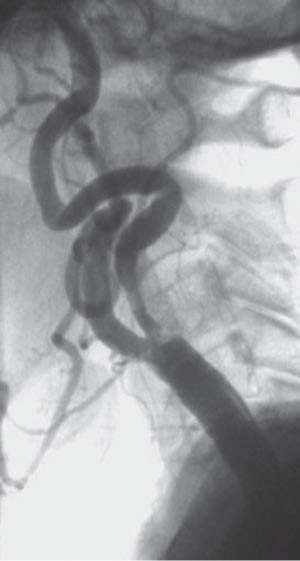
 Inability to advance the EPD distal to the lesion and its deployment in a safe “landing” zone (Fig. 11.1)
Inability to advance the EPD distal to the lesion and its deployment in a safe “landing” zone (Fig. 11.1)
 Inability to safely access the common carotid artery (CCA; such as with severe CCA bifurcation stenosis or external carotid artery [ECA] occlusion with a type III arch)
Inability to safely access the common carotid artery (CCA; such as with severe CCA bifurcation stenosis or external carotid artery [ECA] occlusion with a type III arch)
 Recent (<14 days) moderate to large cerebral infarction
Recent (<14 days) moderate to large cerebral infarction
 Large thrombus burden
Large thrombus burden
 Unfavorable arch anatomy
Unfavorable arch anatomy
 Severe carotid artery tortuosity
Severe carotid artery tortuosity
 Heavy concentric lesion calcification
Heavy concentric lesion calcification
 The “string sign”
The “string sign”
 Preprocedural Imaging
Preprocedural Imaging
 Patient Selection
Patient Selection
| Risk Factor | Features |
Clinical | Age ≥ 80 years |
|
| Decreased cerebral reserve | Prior (remote) large stroke (>1/3 middle cerebral artery territory infarction on CT brain) |
|
| Multiple lacunar infarcts (diffuse lacunes associated with encephalomalacia and/or cerebral atrophy on CT brain) |
|
| Intracranial microangiopathy (CT or MRI brain changes most prominent in the periventricular region) |
|
| Dementia |
Angiographic | Excessive tortuosity | ≥2 90-degree bends within 5 cm of the lesion (including the takeoff of the ICA from the CCA) |
| Heavy calcification | Concentric calcification; width ≥3 mm |
Abbreviations: CT, computed tomography; MRI, magnetic resonance imaging; CCA, common carotid artery; ICA, internal carotid artery.
Excessive vascular tortuosity (Fig. 11.2) and heavy concentric lesion calcification (Fig. 11.3) increase stroke risk through increased manipulation and procedural time,21,22 whereas reduced cerebral reserve decreases brain tolerance to further potential ischemic insult. It must be emphasized that even moderate vascular tortuosity can increase the complexity of the procedure, and presents a hazard for less-experienced operators. Assessing vascular tortuosity in multiple views is essential to fully appreciate the challenge.
Age was demonstrated to be a predictor of adverse outcomes before12 and even after the advent of EPDs.23,24 The ongoing Carotid Revascularization Endarterectomy vs Stent Trial (CREST) lead-in phase showed that octogenarians had a significantly increased risk of adverse events (12.1% vs 3.2% in nonoctogenarians) not accounted for by other factors, and recruitment of these patients was stopped in the lead-in phase.23 The SPACE Trial documented a twofold increase in ipsilateral stroke or death among those >75 years vs ≤75 years (11% vs 5.9%).17 Possible reasons are that increased vascular tortuosity and vessel calcification are more common in elderly patients,25,26 and they are also more likely to have reduced cerebral reserve compared with a younger population.
Recently, however, several investigators have demonstrated that CS can be performed in well-selected elderly patients (≥80 years) with low adverse event rates.25,27,28
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree