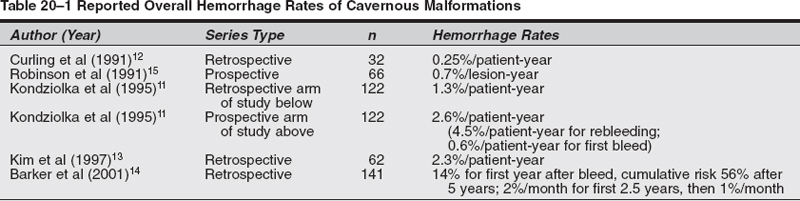
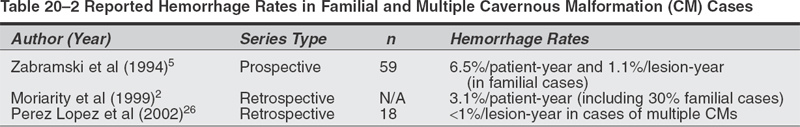
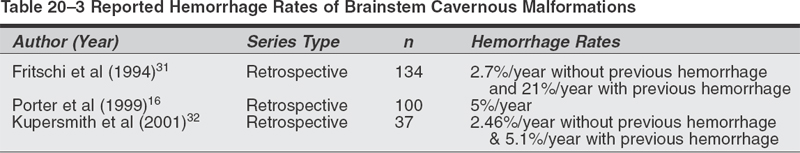
Microsurgical Treatment of Cavernous Malformations Objectives: After completing this chapter, the reader should be able to report options for managing a cavernous malformation patient, and the appropriate indications and approaches for surgical resection. Accreditation: The AANS* is accredited by the Accreditation Council for Continuing Medical Education (ACCME) to sponsor continuing medical education of physicians. Credit: The AANS designates this educational activity for a maximum of 15 credits in Category 1 credit toward the AMA Physician’s Recognition Award. Each physician should claim only those hours of credit that he/she spent in the educational activity. The Home Study Examination is online on the AANS Web site at: http://www.aans.org/education/books/controversy.asp * The acronym AANS refers to both the American Association of Neurological Surgeons and the American Association of Neurosurgeons. Cavernous malformations (CMs), also known as cavernous angiomas or cavernomas, are compact lesions comprised of sinusoidal vascular channels lined by a single layer of endothelium and lacking the full complement of mature vessel wall components. Between the vascular channels in the core of the lesion, there is loose connective tissue stroma without intervening brain parenchyma. Varying degrees of gliotic neural tissue may be found in the periphery of the lesion. The channels are often filled with stagnated blood in varying degrees of thrombosis and degradation.1 The spectrum of cerebrovascular malformations includes arteriovenous malformations, cavernous malformations, venous angiomas, and capillary telangiectasias. In a representative autopsy series, almost 5% of the general population harbored one of these malformations.1,2 Although there is no reliable study which reflects the true incidence and prevalence of CMs in the population at present, the prevalence of CMs has been estimated to be between 0.4 and 0.9% of the population and 8 to 15% of all vascular malformations.1–3 The majority of CMs are located supratentorially. Of the supratentorial CMs, most are located in the white matter of the cerebral hemispheres. The infratentorial CMs are located in the cerebellum, pons, midbrain, and medulla. Less-frequent locations of CMs are the lateral and third ventricles, cranial nerves, and optic chiasm. Acute hemorrhage from a chiasmal CM is a rare cause of permanent visual loss.4 Of the extracerebral locations the cavernous sinus, the orbits, and the spinal cord are the most common. Because CMs are low-flow lesions without arterialized veins or large feeding arteries, they are poorly visualized by angiography and thus labeled “occult.” Computerized tomography (CT) is more sensitive at detecting CMs, but its specificity is low because most appear simply as high-density lesions with little or no contrast enhancement. This is in contrast to the high sensitivity and specificity of magnetic resonance imaging (MRI) for CMs. The MRI appearance of CMs has been categorized into four types: a hyperintense core on T1- and T2-weighted images representing subacute hemorrhage (type I); a “classic” picture of mixed-signal, reticulated core surrounded by a low-signal rim (type II); a iso- or hypointense lesion on T1 and markedly hypointense lesion with hypointense rim on T2, which corresponds to chronic hemorrhage (type III); and punctate, poorly visualized hypointense foci, which can be visualized only on gradient echo MRI, representing tiny CM or telangiectasia (type IV) .5,25 With most asymptomatic CMs, particularly when the diagnosis is relatively clear by MRI characteristics, the right approach for the patient is conservative management with close follow-up, with a few exceptions. In contrast to a bleeding episode from an arteriovenous malformation (AVM), a bleeding episode from a CM is rarely life threatening.6 However, there is more controversy with symptomatic cavernous malformations which hemorrhage in deep, difficult-to-access surgical locations. Here, there are arguments for and against treatment, as well as regarding the type of treatment—open surgery versus stereotactic radiosurgery. The key to making the proper recommendation to the patient is an understanding of the natural history of cavernous malformations. The available data, however, is not as solid as with cerebral AVMs. The rate of hemorrhage for CMs is not as well defined as for AVMs. Several reasons explain this discrepancy. First, some of the published studies dealing with natural history of CMs calculated the retrospective hemorrhage rate, whereas others calculated the prospective hemorrhage rate by using newer databases. The latter studies seem to be more likely to reflect the true risk of hemorrhage from a CM, but their period of follow-up is understandably shorter. The second reason is the definition of hemorrhage in published series in the literature. Hemorrhage from a CM may not always be identifiable clinically or radiologically in contrast to hemorrhage from an AVM. This is because there is no uniform pattern of bleeding from CMs, nor is there homogenous terminology to define hemorrhagic episodes. A report suggested that radio-logically defined hemorrhage might miss clinically significant events. It therefore proposed the use of “event rates”—defining neurological deterioration that is experienced by the patient as a subjective worsening, and associated with objective worsening of clinical status, independent of the radiological findings. A study described the three patterns of hemorrhage from CMs: “slow ooze” producing the hemosiderin ring seen on MRI, “intralesional hemorrhage” producing an expansion of the lesion on MRI with or without subtle increase in symptomatology, and “gross hemorrhage” producing acute severe symptoms and intra- or extralesional acute hemorrhage on MRI.8 Third, the clinical importance of hemorrhage from a CM is dependent not only on the severity of hemorrhage but also on the location of the CM in the brain. For example, a moderate-size intracerebral hemorrhage (ICH) in the nondominant anterior frontal lobe may not cause any neurological symptoms that may warrant radiological work-up, while even a small intralesional hemorrhage from a CM in the brainstem or optic chiasm may produce significant disability. Epilepsy and focal neurological deficits were found to be the most common symptoms in a series of 30 children with an average age of 9.4 years.9 A study of 36 children demonstrated higher hemorrhage risk and lower incidence of epilepsy—which may be related to chronic or recurrent microbleeding—when compared with adults.10 A retrospective study calculated an annual hemorrhage rate of 1.3% per patient-year11; a second study12 calculated a rate of 0.25% per patient-year12; and still a third study reported a rate of 2.3% per patient-year13 A prospective study reported a 0.7% hemorrhage rate per lesion-year.15 Another prospective study of 11 patients with CMs reported an overall hemorrhage rate of 2.6% per patient-year.11 Rebleeding rate in patients with previous hemorrhage from their CMs was 4.5% per year, in contrast to a bleeding rate of 0.6% in patients without prior evidence of hemorrhage. In a retrospective review of 141 patients with CM and previous hemorrhage, the cumulative incidence of a second hemorrhage was 14% after 1 year and 56% after 5 years; during the first 2.5 years after a hemorrhage, the monthly rehemorrhage rate was 2%, and the risk then decreased spontaneously to less than 1% per month, a 2.4-fold decline.14 Rehemorrhage rates were higher in younger patients. Table 20-1 summarizes the overall hemorrhage rates found in published case series. The actual population hemorrhage rates likely lie between the lowest retrospective and the highest prospective rates. Patients with asymptomatic lesions have traditionally included those presenting with mild headache and nonspecific symptoms. Although there is insufficient data to precisely identify which patients progress to develop symptoms or the risk factors associated with symptomatic transformation, several authors have implicated the following putative “predisposing” factors.6 A report mentioned that the hemorrhage risk may be higher in females and in CMs located in the brainstem. Others also found that females have a higher risk of hemorrhage. In one study, estrogen receptors were demonstrated in a few CMs in females.16 Immunohistochemical studies demonstrated the presence of proliferating cell nuclear antigen and angiogenic growth factors (vascular endothelial growth factor, basic fibroblast growth factor, and transforming growth factor a), suggesting that the endothelium of CMs is not static but it proliferates.17–19 These findings led some authors to hypothesize that CMs should be classified as slowly growing vascular neoplasms rather than true developmental vascular malformations.17–19 Familial cases of cavernous malformations have been recognized to constitute 30 to 50% of all cases, although a referral bias may be responsible for exaggerating this frequency.20 Familial cavernomas are believed to be transmitted as an autosomal dominant trait. The gene Kritl, located at the CCM1 locus and responsible for familial cavernomas, has been localized to the long arm of chromosome 7.21–24 It was initially characterized in patients of Hispanic descent.20 Compared with sporadic cases, familial patients more often harbor multiple lesions. In a series of familial cases only, 84% of patients had multiple lesions versus 10 to 15% of patients in sporadic cases.5 A French study of 33 asymptomatic patients diagnosed with familial cerebral CMs followed prospectively showed appearance of a new lesion—de novo formation—in 30.3% of patients, confirming the dynamic nature of familial CMs as a hallmark of this variant.25 A report observed a 3.1% symptomatic hemorrhage rate per patient per year in a series of eight patients with 30% familial cases.2 A study of only familial CMs reported a prospective hemorrhage rate of 1.1% per lesion-year (6.5% per patient-year) .5 The disparity in this familial series between lesion and patient hemorrhage rates underscores the high frequency of multiple lesions in familial cases. A study of 18 cases of multiple CMs and 200 lesions determined a hemorrhagic rate per lesion per year of <1%.26 A Japanese case report described a patient not only with multiple congenital CMs but also with a thrombosed cerebral AVM.27 Evaluation of congenital vascular anomalies needs to take into consideration the combination of other congenital vascular anomalies and their familial occurrence. Table 20-2 summarizes the hemorrhage rates for familial CMs and cases of multiple CMs already reported in the literature. Sporadic and familial forms of these lesions behave similarly, or there is insufficient data to conclusively show a difference2. Cavernous malformations of the brainstem constitute a special entity and pose a challenge to neurosurgeons dealing with these lesions for different reasons. In view of their location, they rarely present incidentally. They produce more severe symptoms and neurological deficits than CMs in other locations. Clinically, these patients generally present with an ictus. The most common symptoms are headache, vertigo, nausea, and diplopia. The most common focal deficits are palsies of cranial nerves III to VII. One case report described cerebellar mutism after hemorrhage from a midbrain CM.28 Another anecdotal report described a patient presenting with acute hydrocephalus from a hemorrhagic tectal CM.29 Two cases of hemorrhagic brainstem CMs causing vestibular and auditory symptoms, such as sudden deafness, fluctuating hearing loss, and Meniere-like vertigo, have been reported.30 In a series of 41 patients and a review of another 98 cases from the literature, none of the brainstem cavernomas presented as an incidental finding.31 Second, there is evidence in the literature that the hemorrhage rate of brainstem CMs is greater than in other locations. A series of 100 patients with CMs of the brainstem found a 5% per person annual risk of hemorrhage.16 Of 12 patients who were followed-up without surgical intervention for an average of 35 months, 7 were in the same condition or better (58%), 4 were worse (33%), and one died. Another study reported a 2.7% annual rate of hemorrhage in patients without previous hemorrhagic events, and 21% in those with previous hemorrhage.31 A retrospective review of 37 patients32 yielded a bleeding rate of 2.46% per year with brainstem CMs and a rebleeding rate of 5.1% per year; younger patients <35 years old and CMs > 10 mm in diameter showed a greater propensity to bleed. The problem here is that this higher rate in the brainstem may be because hemorrhages in this location are usually symptomatic and therefore easier to identify than hemorrhage from lesions in less eloquent locations. Table 20-3 summarizes the bleeding rates found in previously published reports for brainstem CMs. Particularly in the brainstem, multiple bleeding episodes may increase the likelihood of a persistent neurological deficit. In the series of Porter et al, rebleeding caused debilitating and persistent deficits in 50% of patients with CMs of the brainstem and thalamus.16 There are only rare reports of thalamic CMs. One Italian study examined the clinical course, treatment, and outcome of 12 patients with symptomatic thalamic CMs.33 Sixty-six percent of patients presented with intracerebral hemorrhage; four patients underwent radical surgery, resulting in one death, one patient without change in neurological status, and two improved patients. One patient underwent radiosurgery that resulted in rehemorrhage 4 months after the procedure. Five patients underwent conservative treatment, with a 40% rate of rebleeding within 2 years. Patients with lesions in the region of the third ventricle presented with a more insidious onset of symptoms related to their mass effect rather than an acute hemorrhagic episode. None of these lesions were identified incidentally.34,35 Outcome is related to premorbid status, and preoperative cognitive difficulties are unlikely to resolve after surgery. Given the nature of the location of these lesions—suprachiasmatic region, the region of the foramen of Monro, the wall and floor of the third ventricle—surgery carries a significant risk of at least transient endocrinologic or neurological dysfunction.2 Ventricular location is rare for CMs. Only 10 pediatric cases out of 46 previous cases of intraventricular CMs have been published.36 Imaging features differ from intraparenchymal sites. Diagnosis is made only by histopathological examination due to the lack of classic magnetic resonance image (MRI) findings.37 A descriptive study found four modes of clinical presentation in patients with CMs of the spinal cord.38 These presentations relate directly to the degree of extralesional hemorrhage. Patients may present with an acute onset followed by a rapid decline, an acute onset followed by a gradual decline, a slow progressive decline, or discrete episodes of decline with intervening remission. The latter clinical picture of exacerbations and partial or complete remissions is also seen with CMs of the brainstem and accounts for the fact that CMs of the spinal cord and/or brainstem are frequently initially misdiagnosed as multiple sclerosis. The symptoms of spinal cord CMs generally consist of a painful myelopathy progressing to paraparesis or paraplegia.39 As with cavernous malformations in the region of the third ventricle, there is little in the literature discussing hemorrhage rate in these lesions. Outcomes in the most recent series are good, with almost all patients showing improvement in their symptoms following surgery.38,3 Most authors advocate surgery for symptomatic patients and believe that postoperative outcome is related to preoperative status.38,39 These authors also advocate following lesions found incidentally in asymptomatic patients. The majority of these lesions grows within the cavernous sinus and expands it, stretching the third, fourth, and fifth cranial nerves over the pseudocapsule of the CM. The sixth cranial nerve is usually contained within the substance of the malformation.40 Patients present with the acute or subacute onset of visual symptoms such as diplopia, ptosis, exophthalmos, and visual acuity disturbances. In contrast to cerebral CMs, these lesions do not have a characteristic MRI appearance. However, a study reported MRI features of CMs of the cavernous sinus that may help in differentiation from meningiomas, such as a dumbbell shape with a small part in the suprasellar region and a large part in the cavernous sinus, as well as higher signal intensity on T2-weighted images.41 Conservative management does not necessarily mean observation only. In some cases, microsurgical resection may actually be considered a more conservative route than expectant observation. Supratentorial Cavernous Malformations Surgical management does not appear to be indicated for the control of seizures unless intractable in nature. However, if a patient has a new-onset seizure and workup reveals a CM associated with a large hematoma, the most conservative approach may be to allow the hemorrhage to resorb, and once the patient is neurologically stable to excise the lesion.42 Brainstem Cavernous Malformations There are two approaches to symptomatic brainstem CMs. For a first-time bleed, the most conservative approach is to follow the patient clinically. If the patient has a second neurological event from hemorrhage and the lesion is surgically accessible, then surgical excision may very well be the most conservative approach. Multiple Cavernous Malformations Multiple CMs should be treated as if the symptomatic lesion was the only lesion. Although there may be simultaneous bleeding in two or more lesions, surgical treatment should be undertaken for lesions jeopardizing critical structures or exerting mass effect.43 All other lesions are to be followed expectantly like any other incidental CM. Spinal Cord Cavernous Malformations The potential for neurological devastation from a hemorrhage is very high. The question of whether this high potential warrants prophylactic surgery as the most conservative approach remains unanswered in clinical studies and anecdotal reports. Indications The indications for microsurgical resection of supratentorial CMs are outlined by Shah and Heros44: (1) progressive neurological deficit, (2) documented episodes of recurrent hemorrhage, (3) medically intractable epilepsy, and (4) the need for tissue to establish a pathological diagnosis. In summary, accessibility, certainty of diagnosis, history of recurrent hemorrhage, and significance of focal neurological deficit are the main factors to weigh in the decision of whether to recommend surgical resection or not. A mathematical analysis of surgical decision-making in cerebral CMs showed that for superficial lesions, permissible surgical risk ranged from 0.4 to 2.8% of combined morbidity and mortality.45 The surgical gain of morbidityfree life expectancy was small (0 to 1.1 years). For deep lesions, permissible risk of surgery was larger, 64.1% for 20-year-olds and 31.4% for 60-year-olds. The gain in morbidity- free life expectancy was 17 to 35 years for 20-yearolds, but only 1.1 to 3.1 years for 60-year-olds. Hence, surgery is mathematically justified for younger patients with deep lesions. Technical Pearls The MRI is the principal imaging modality with its multiplanar capability. It is most helpful in planning operative approaches. There are limited indications for preoperative angiography: (1) extracerebral location, (2) hemorrhagic presentation, or (3) an atypical MRI or computerized tomography (CT) scan’s appearance.46 Stereoscopic angiography may help to identify sulci that can be used to approach deep lesions and minimize transcortical dissection. It is desirable to utilize intraoperative electrophysiologic functional mapping if the lesion is large and deep. Surgical resection of supratentorial CMs in accessible areas is relatively straightforward, as there is no major arterial supply and subsequently no major blood loss. The most difficult part of the case, if any, is intraoperative localization. In addition to one’s knowledge of topographic sulcal/gyral anatomy, the surgeon can usually be aided by CT- or MRI-based stereotactic guidance or intraoperative ultrasound. A trans-sulcal approach is advocated by many because of its ability to minimize cortical resection.47–50 However, whether disruption of the U-fibers in the trans-sulcal approach is less detrimental than disruption of the vertical fibers in the trans-gyral approach remains unknown.49,51 The CM is grossly identified as a well-circumscribed, blue or purple, lobulated mass resembling mulberries or grapes. As previously stated, bleeding is not a major problem. Occasionally, there are small feeding arteries that course with the sinusoidal spaces at the periphery of the lesion.52 A well-defined gliotic plane allows for easy separation and complete removal of the lesion from the surrounding white matter; incomplete removal of the lesion incurs the risk of rebleeding that should be avoided. The hemosiderin-laden gliotic tissue comprising the capsule of the CM is epileptogenic and should be removed if not located in eloquent neural tissue.53 Overall Postoperative Outcomes Bertalanffy et al reported excellent results and acceptable morbidity in a series of 72 patients with CMs surgically excised over 5 years.3 There were 24 brainstems CMs, 18 in the deep white matter, 12 in the basal ganglia or thalamus, 11 supratentorial, and 7 in the cerebellar hemispheres. The perioperative morbidity was reported at 29.2%; however, the long-term morbidity fell to 5.5%. There were no mortalities is this series. In another surgical series of 35 patients with 21 hemispheric CMs, 4 intraventricular, 4 brainstem, and 6 cerebellar, complete excision was achieved in 33 patients, with good outcome defined as improved seizure control or neurological deficit in 34 patients.54 In a surgical series of 47 patients with CMs, only one mortality was reported with all other patients reaching satisfactory results—no recurrent hemorrhage, seizure-free survival, and low morbidity.55 A pediatric surgical series of 24 children with cerebral CMs yielded good postop results, with one child that died and nine with persistence of preoperative neurological deficits.56 An Italian surgical series of 74 cases and 76 CMs (57 hemispheric, 4 intraventricular, 1 middle cranial fossa, 2 brainstem, 5 cerebellar, and 7 orbital) reported good outcome in 66 patients, resulting in improved seizure control or lessened neurological deficit, and mortality in 2 cases.57 Postoperative Outcomes in Cavernous Malformations of the Cavernous Sinus Surgery of CMs of the cavernous sinus carried a 36% mortality rate because of excessive bleeding. In fact, although histologically these cavernous sinus lesions look like typical CMs, at surgery they behave more like hemangioblastomas with a potential for catastrophic bleeding if the lesion itself is entered without previous maneuvers aimed at decreasing their vascularity, such as direct intralesional embolization. In 27 of the 53 cases reviewed, outcome for cranial neuropathies was analyzed. Twelve patients showed worsening after surgery, 11 patients showed improvement, and 4 patients showed no change.40 In another series of 13 patients who underwent an extradural approach for resection of CM of the cavernous sinus, there was no recurrence or growth of the residual lesion; however, the outcome of extraocular movements was poor.58 Considering the benign nature of these lesions and the disability associated with an irreversible cranial neuropathy, less aggressive management may be advisable. Table 20-4 summarizes the case series that provide either support for or against microsurgery for supratentorial CMs. Postoperative Outcomes of Epilepsy and Cavernous Malformations In a large series, 78.7% of patients showed good concordance between location of cortical CM and site of seizure focus. In cases of good concordance, complete lesionectomy resulted in disappearance of seizures.59 Robinson et al reported a series of 32 patients with severe seizure disorder; 18 patients had medically intractable seizures.15 Fourteen patients with seizures and an associated CM underwent surgical resection. Unfortunately, 50% continued to have seizures postop. A similar percentage and outcome was reported by McCormick et al.60 There still are anecdotal reports of good control of seizures following surgical excision of CMs in patients with previously uncontrolled seizures.61 Three patients undergoing surgical treatment for intractable epilepsy with CM in the dominant hemisphere had good results with a seizure-free postoperative course and no language or cognitive deterioration.62 Another two patients with long-standing medically intractable epilepsy and CM were treated surgically, where the lesion and surrounding epileptogenic tissue were removed, resulting in seizurefree survival.63 In a German series, 14 patients were operated on for CMs with seizures at presentation. Twelve of 14 patients improved; 10 of 14 had complete relief of epilepsy.64 A series of 11 children undergoing surgical removal of CM causing epilepsy led to eight patients becoming seizure-free on the same preoperative drug therapy, one seizure-free on reduced drug dosage, and two seizure-free on no drug therapy.65 A retrospective series of 36 patients suffering from epilepsy from cerebral CMs undergoing surgical treatment showed a complete cure in 25%, improved seizure control with decreased medication in 30.5%, and improved seizure control on the same preoperative drug regimen in 44.5%.66 Table 20-4 Published Series for and against Microsurgery for Supratentorial Cavernous Malformations For Against Bertalanffy et al (2002)17 Attar et al (2001)54 Mahlah et al (1999)55 Acciarri et al (1993)57 * Pediatric series.

 Natural History and Clinicopathological Features
Natural History and Clinicopathological Features
 Familial Cavernous Malformations
Familial Cavernous Malformations
 Cavernous Malformations of the Brainstem
Cavernous Malformations of the Brainstem
 Cavernous Malformations of the Thalamus
Cavernous Malformations of the Thalamus
 Cavernous Malformations of the Third Ventricle
Cavernous Malformations of the Third Ventricle
 Cavernous Malformations of the Lateral Ventricles
Cavernous Malformations of the Lateral Ventricles
 Cavernous Malformations of the Spinal Cord
Cavernous Malformations of the Spinal Cord
 Extra-axial Cavernous Malformations
Extra-axial Cavernous Malformations
 Conservative Management
Conservative Management
 Microsurgery for Supratentorial Cavernous Malformations
Microsurgery for Supratentorial Cavernous Malformations
†Series of cavernous malformations of the cavernous sinus.
Cavernous Malformations
Only gold members can continue reading. Log In or Register to continue

Full access? Get Clinical Tree


