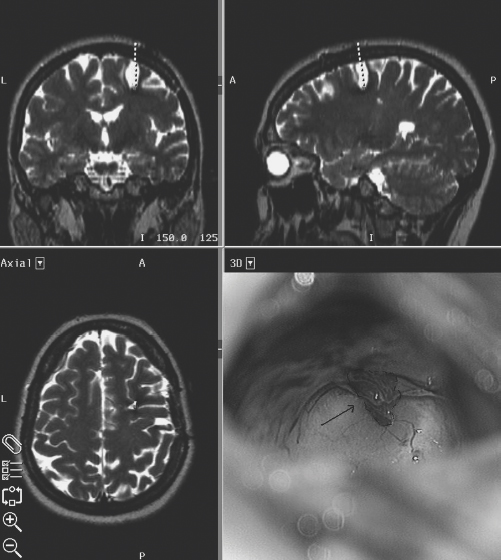
15 Giovanni Broggi, Paolo Ferroli, and Angelo Franzini Cavernous malformations (CMs) are an increasingly recognized cause of partial epilepsy. These benign, mulberry-like vascular lesions may occur at any site within the CNS, as well as in other organs such as the liver, bone, or skin. Histologically, they consist of ectatic, endothelium-lined channels without mural muscular or elastic fibers within a matrix of collagenous tissue lacking any neuronal elements. Typically, although not invariably, gliosis and hemosiderin deposition can be found in the surrounding neural parenchyma.1 Supratentorial CMs present with epileptic seizures, less often with hemorrhage or with signs and symptoms of space-occupying lesions.2–6 The diagnostic incidence of CMs has dramatically increased in the magnetic resonance imaging (MRI) era. It is not uncommon to see patients with incidentally diagnosed, asymptomatic CMs.7–9 Epileptic seizures caused by CMs are often medically refractory10,11 as is often the case for partial seizures secondary to other space-occupying lesions.12,13 Even in patients who present with seizures as the first clinical manifestation, MRI often shows the presence of microhemorrhages. The presence of microhemorrhages in patients with CMs who present with seizures at the first clinical manifestation has been confirmed by postoperative histologic analysis.8,9,14 Epileptic seizures are thought to be the consequence of hemosiderin deposits or the presence of gliotic scars secondary to the microhemorrhages.14,15 Surgical treatment of CMs presenting with seizures is usually recommended, not only to prevent future bleeds but also to prevent future seizures.16 However, surgical indications and optimum management of CMs causing epilepsy still remain controversial.11,16–24 In cases of intractable epilepsy with concordant clinical, electrophysiologic, and neuroimaging findings, the indication for surgery is clear. There are no accepted guidelines for the management of patients with CMs and recent onset of seizures or with medically controlled seizures. The best surgical strategy in patients with CMs presenting with seizures (i.e., simple lesionectomy versus “epilepsy surgery”), is also debated. Of 191 patients (111 men, 80 women) with intraparenchymal hemispheric CMs who underwent surgical treatment at our institute between 1988 and 2003, 163 (85.3%) presented with seizures. The mean age at the time of surgery was 33.4 ± 14.2 years (range, 17 to 63 years). The mean duration of illness was 4.5 ± 7.6 years (range, 15 days to 43 years). Ninety-nine (60.7%) patients had a history of chronic epilepsy and a longer mean duration of illness (10.2 ± 9.1 years). Sixty-four (39.3%) patients had only single or sporadic seizures, and an immediate diagnosis of CM and surgical treatment, so that the mean duration of illness was much shorter (1.2 ± 1.7 years). In patients with chronic epilepsy, the aim of the preoperative investigation was to identify a possible correlation between electroclinical and anatomic data. Data from detailed clinical histories, neurologic examinations, MR images, and scalp electroencephalograms (EEGs) were collected and examined. Seizures were divided into simple partial, complex partial, and secondary generalized seizures according to the International League against Epilepsy classification. Scalp EEGs with hyperventilation and photic stimulation are obtained by use of 16- or 18-channel bipolar recordings according to the International 10–20 system. In some cases, sleeping and waking EEGs were recorded. Only a few patients required video-EEG recording. EEGs were classified as normal; nonspecific, when nonfocal waves were present; or focal, when slow waves, sharp waves, spikes, rapid activity, or any combination of these abnormalities were restricted to one or two adjacent channels. All patients underwent preoperative MRI (0.5 or 1.5 T). Magnetic resonance images consisted of multiplanar spin echo sequences using T1- and T2-weighted images and, also, in most patients, gradient echo T2-weighted images. Functional MRI data were collected in patients with CMs in eloquent areas treated more recently. When a correlation between cavernoma location and electroclinical data was found, patients underwent lesionectomy without any other investigation. Only patients with incongruent data (multifocal seizures, multiple cavernomas, suspected dual pathology, etc.) were considered candidates for further invasive studies. When lesionectomy alone was performed, this was accomplished by a minimally invasive transsulcal approach under high magnification. Before neuronavigation became available, the entrance sulcus was chosen with the help of a stereotactic frame. A guidance catheter was inserted to guide the surgeon only when approaching deep lesions. Frameless image-guidance with different neuronavigation systems has been employed since 1995. Functional MRI data fused with conventional neuronavigation MR images and direct cortical mapping data collected during awake surgery were used for surgical planning in case of eloquent location. Short linear skin incisions (6 to 8 cm) were generally used. The diameter of the craniotomy (2 to 4 cm) was chosen according to the amplitude of the arachnoidal incision planned to reach the lesion. The larger and deeper the lesion is, the longer the arachnoidal incision. This strategy was adopted to avoid any traction at the edges of the arachnoidal incision and to minimize compression on the cortical surface exposed within the sulcus. Retractors were generally avoided; when used, care was taken to keep them loose. The cortical surface within the sulcus is protected with unsticky cottonoids. Sharp incision of the superficial arachnoidal layer and underlying arachnoidal bands under high magnification is used (the tip of a 22-gauge needle used as a knife and microscissors). Any damage to the pial surface of the surgical corridor to the lesion is carefully avoided, until the pial surface covering the lesion is reached and incised using bipolar low-current coagulation. Particular care is taken to avoid damage to the vessels at the bottom of the sulcus. The surgical strategy is lesionectomy, limiting the removal to the CM and covering cortex, as clear evidence is lacking that better outcomes result from removing the hemosiderin-stained gliotic perilesional tissue. Postoperative follow-up included similar clinical, neuroradiologic, and electroencephalographic examinations used in the preoperative assessment. Antiepileptic drugs in patients with seizures were withdrawn after at least a 1-year seizure-free follow-up period. Three patients required reoperation because of recurrent seizures due to residual CM (Fig. 15-1). The mean duration of follow-up was 48 months (range, 0.5 to 14 years). Figure 15-1 Surgical plan and intraoperative view of a case of residual cavernoma (arrow and dotted line) that required surgery for seizure persistency. After repeated surgery, the patient was seizure-free. None of the patients had any risk factor for epilepsy. Preoperative neurologic examination was within normal limits in 139 patients. The other 24 had focal neurologic signs. In one patient who had suffered seizures since the age of 13, mild hemiparesis had become apparent in the early months of life: this was believed to be secondary to the presence of a giant motor strip CM. Two patients suffered intracerebral bleeding after having presented with seizures. Mesiotemporal, temporolateral, or insular cavernomas were more commonly observed in patients with chronic epilepsy (mesiotemporal, 19.2%; neocortical temporal, 34.3%) than in those with occasional seizures (mesiotemporal, 7.8%; neocortical temporal, 17.2%); the most frequent location in this latter group being the frontal region (57.6%, versus 19% of the patients with chronic epilepsy). The CM was subcortical in 52.1%, cortical in 19.6%, and corticosubcortical in 12.3% of cases. There were no significant differences in cortical or subcortical location in patients with chronic epilepsy when compared with those with occasional seizures. The size of the lesions ranged from 0.5 to 4 cm, although in one patient, the CM was unusually large (6 cm) and mimicked a hemorrhagic tumor. The MRI findings reproduced the typical picture of CMs previously reported.9,19 The core of the malformation was commonly found to have a high signal in both T1- and T2-weighted images, thus indicating the presence of extracellular methemoglobin. In some patients, mixed areas of decreased and increased intensity were observed, the result either of different stages of hemorrhage or of interspersed areas of calcification. In all of the patients, T2-weighted images revealed peripheral marginal or ring-like hypointensity caused by hemosiderin drift. Eleven patients had evident radiologic signs of previous bleeding with intraparenchymal hematoma, for which three of them had undergone surgery in the years preceding the excision of the CM. An associated developmental venous anomaly was commonly observed. Mass effects and surrounding edema were found in 14 patients. Twenty-two patients had multiple CMs (13.5%). In the 99 patients with chronic epilepsy, seizures were completely controlled by antiepileptic drugs in only 36 patients. Thirty-seven of the 163 patients had exclusively generalized tonic-clonic seizures. Twenty-two patients had usually generalized tonic-clonic seizures with focal onset. Simple partial seizures were reported in 64 patients and partial complex seizures in 35 patients, with simple onset in 11. In two patients with multiple CMs, two different types of seizures were reported. The results were normal or nonspecific in 40.9% of those who had waking EEGs; 61.1% of the patients also had sleep EEGs, which were normal in 45.4% and revealed focal abnormalities in 54.6%. Overall focal electroencephalographic abnormalities were found in 68.2% of the total patient population. Among the 99 patients with chronic epilepsy, 68 (68.7%) are completely seizure-free (20 of 68 [29.4%] still under AEDs [antiepileptic drugs]), 10 (10.1%) have only sporadic seizures, and 17 (17.1%) still have seizures despite surgery and therapy. Four patients were lost to follow-up. In patients with preoperative drug-resistant epilepsy, only 60% were seizure free at follow-up. As previously mentioned, three patients required reoperation because of seizures due to residual CM (Fig. 15-1). Sixty-three of the 64 (98.4%) patients without chronic epilepsy (single or sporadic seizures) were completely seizure free (28% still waiting for definitive withdrawal of AEDs, which usually occurs 2 years after surgery). One patient was lost to follow-up. A longer clinical history of chronic epilepsy was found to be related to a poorer prognosis. No clear correlation between CM location and outcome could be found even though there was a trend for mesiotemporal CMs to have poorer prognosis in terms of seizure control. Postoperative focal neurologic signs (sensorimotor defects and homonymous hemi- or quadrantopia) appeared in 12% of patients. Most of these signs were transient and had completely disappeared or were greatly reduced at subsequent clinical examinations. Only in three (1.8%) patients was a partial residual deficit found at long-term follow-up. These consist of slight hand paresis in a patient with a CM under the motor hand area; right inferior limb paresis with a slight gait impairment in a patient with a CM of the mesial prerolandic cortex; and hemianopsia in a patient harboring a CM in the depth of the calcarine scissure who required emergency surgery for evacuation of a postoperative hematoma. There was no mortality. Postoperative MRI was available in 122 cases and showed a complete CM resection in all patients (in three after repeated surgery). Hospital stay and duration of surgery progressively shortened as image-guided minimally invasive techniques became available. Mean surgery duration in the last 50 cases was around 2 hours and the mean hospital stay 4 days. The underlying mechanisms causing seizures in patients with CMs are complex and still not completely understood. Given the lack of intralesional brain tissue, CMs per se are clearly not epileptogenic.25 Furthermore, the mass effect does not explain the high epileptogenicity of this vascular malformation. Other lesions of larger size such as diffuse growing malignant tumors are less commonly associated with medically refractory epilepsy,26,27 and epileptogenic mechanisms seem to be different.28 Awad and Robinson compared the seizure incidence in patients harboring a CM with that of patients with arteriovenous malformations (AVMs) or gliomas and found an incidence of 50 to 70% in cavernomas, 20 to 40% in AVMs, and 10 to 30% in gliomas.29 In our series, 99 of 163 (60.7%) patients operated on for a CM were affected by chronic epilepsy. Seventy-seven of 99 (77.7%) were drug resistant. Del Curling et al. estimated the risk of developing seizures at 1.51% per person/year and 2.48% per lesion/year for those with multiple lesions.30 According to Cohen et al., 41 to 59% of symptomatic CMs will present with seizures,31 and around 4% of refractory partial epilepsies are thought to be symptomatic of CMs.32 Williamson et al. recently reported the results of intracellular recording from neurons adjacent to intracerebral neoplasms and CMs.28 Neurons adjacent to CMs were found to have a greater propensity to show large, complex, spontaneous synaptic events than neurons adjacent to tumors. Both spontaneous excitatory and inhibitory events were recorded. Neurons neighboring CMs displayed more excitable responses to synaptic stimulation, with multiple action potentials riding on prolonged excitatory postsynaptic potentials being evoked (71% vs. 32% of neurons from the tumor group). In studies using hippocampal tissue, these authors noted a similar pattern of spontaneous activity in tissue adjacent to CAs, suggesting that a common synaptic mechanism should be hypothesized for both neocortical and hippocampal CMs. The prevalence of epileptiform responses appeared to correlate only with the proximity of the lesion. The underlying cause for CM epileptogenicity is thought to be the presence of chronic, clinically silent microhemorrhages25,33 secondary to fragility of the capillary sinusoidal wall and lack of tight junctions. This results in deposition of iron-containing blood breakdown products such as hemosiderin, a probable degradation product of ferritin, as well as hemin, a globin breakdown product, in the adjacent brain tissue. Iron salts are proven potent epileptogenic agents when applied on the rat cortex.32,34,35 Iron may generate epilepsy by different mechanisms. As an electron donor, iron is implicated in the production of free radicals and lipid peroxides, which interact with receptor activity, calcium channels, cellular transport proteins, intracellular second messengers, and neurotransmitter (glutamate and aspartate)-mediated excitotoxicity.33,36,37 Von Essen et al. found a marked increase in the levels of serine (fivefold), glycine (10-fold) and ethanolamine (20-fold) in the peripheral zone of cerebral CMs.38 In addition, iron deposition seems to inhibit glutamate uptake. Such biochemical abnormalities in the marginal zone of CMs may cause excessive activation of excitatory transmission. Studies using a ferrous chloride model of epilepsy demonstrated that gliosis and neuronal loss can occur39 presumably because of the generation of free radicals and subsequent lipid peroxidation.40,41 Iron-triggered cellular alterations are more likely and more significant the longer the duration of epilepsy. As a consequence, the tissue adjacent to CMs becomes increasingly epileptogenic.42 This can explain why the longer the history of epilepsy, the poorer are the results of pure lesionectomy. Iron-laden epileptogenic tissue may cause independent secondary epileptogenic foci in experimental animals by kindling. However, it is controversial whether or not such secondary foci are found in humans. One indicator of secondary epileptogenesis in humans is the finding of dual pathology, that is, hippocampal neuronal cell loss in some patients harboring extrahippocampal lesions such as brain tumors, cortical dysgenesis, or vascular malformation.27,43–45 In patients with CMs, dual pathology has rarely been found.28,43,46 In these patients, lesionectomy did not result in seizure control, and subsequent resection of the mesial temporal lobe structures became necessary to achieve satisfactory seizure outcome.43,46,47 In summary, as far as epileptogenesis of CMs is concerned, it can be speculated that the hemosiderin deposition near CMs results in impaired glutamate uptake as well as injury-induced synaptic reorganization, which may subsequently allow neuronal hypersynchronization in focal regions. This can then propagate activity to more distant regions. Surgical indication should arise from the comparison between the risks of surgery and the risks related to the natural history of these lesions. As far as CMs are concerned, there has been in the recent past a growing tendency to recommend resection of supratentorial CMs for the following reasons: It is our policy to recommend surgery for all symptomatic supratentorial CMs. Many retrospective studies reported a good outcome after CM resection in patients with seizures (Table 15-1). Seizure outcome of lesionectomy alone is excellent with improvement of seizure control in 92% of cases, amounting to abolishment of seizures in 84%.51 No data are available to clearly demonstrate a different outcome if lesionectomy includes the perilesional hemosiderin-stained tissue. In our opinion, there is no significant difference in seizure outcome whether lesionectomy alone or a “seizure operation” is performed. Literature data suggest that seizure history and length of clinical history are the main prognostic factor. This confirms what Olivecrona and Riives stated as early as 1948: “… the prognosis of epilepsy is best in the case of the younger person with a short history of epilepsy, while in cases of inveterate disease with a long history of epilepsy the outcome is poor.” In our series, all patients who underwent lesionectomy after the first seizure or who presented only with sporadic seizures remained seizure-free at long-term follow-up, whereas 40% of patients with a clinical history of chronic epilepsy (often drug-resistant) still had seizures after lesionectomy. Early lesionectomy seems therefore to be the best way to avoid the development of chronic epilepsy. The fact that the duration of epilepsy at time of surgery has a negative influence on seizure outcome underscores the importance of early CM resection. It is our policy to recommend surgery after the first seizure. What to do when a chronic, often drug-resistant epilepsy develops still remains under debate. Lesionectomy obviously holds the greater theoretical benefit of removing the smallest amount of nonpathologic cerebral parenchyma by means of a straightforward, low-risk, minimally invasive surgery, requiring a few days of hospitalization. Such a minimal resection, however, may not provide the desired complete relief from seizures. Conversely, localization and removal of the epileptogenic zone may provide a higher rate of cure than lesionectomy alone. The price of real epilepsy surgery may include invasive electroclinical investigations such as stereoelectroencephalography, corticography, and strips recording of brain parenchyma outside the lesion. The surgical resection may be tailored according to electroclinical data and may result in a considerably wider resection than pure lesionectomy. In our series, lesionectomy alone allowed for the control of seizures in 60% of epileptic patients, avoiding potential complication of invasive studies, subsequent resection of brain parenchyma, along with added time and equipment costs. These considerations led in our institute to the institution of a two-step surgery for CM-related chronic epilepsy. Patients and families are fully informed, and during the first operation only the lesion is removed. Twelve to 24 months later, if drug-resistant seizures are still present (despite MRI demonstration of radical resection), second surgery is performed with the goal of removing some of the surrounding hemo-siderin-stained brain often with the guidance of invasive electrophysiologic data.
Cavernous Malformations and Seizures: Lesionectomy or Epilepsy Surgery?
 Surgical Treatment at Instituto Nazionale Neurologico Carlo Besta
Surgical Treatment at Instituto Nazionale Neurologico Carlo Besta
Preoperative Assessment
Surgical Technique
Postoperative Follow-up
Clinical History, Preoperative Neurologic Examination, and Cavernous Malformation Location
Epilepsy Outcome after Lesionectomy
Mortality and Morbidity
 Epileptogenesis in Patients with Cavernous Malformations
Epileptogenesis in Patients with Cavernous Malformations
 Indications for Surgical Treatment
Indications for Surgical Treatment
 Lesionectomy or Epilepsy Surgery?
Lesionectomy or Epilepsy Surgery?
| Author (Year) | Number of Cases | Outcome |
|---|---|---|
| Lonjon et al. (1993)3 | 16 | 14/16 seizure-free 2/16 improved |
| Giulioni et al. (1995)16 | 11 | Improved seizure control in 100% Seizure-free without therapy: 18% |
| Zevgaridis et al. (1996)23 | 168 | 88.3% seizure-free 6.5% marked reduction in seizure frequency |
| Braun et al. (1996)17 | 14 | 10/14 complete relief 2/14 improved |
| Cappabianca et al. (1997)18 | 35 | Less than five preoperative seizures:100% seizure-free More than five preoperative seizures: 62.5% seizure-free |
| Moran et al. (1999)51 | 33 | Improvement in seizures in 92% |
| Mahla et al. (1999)4 | 31 | Alleviation of epilepsy: 21/31 Seizure-free without medication: 4/31 |
| Current series (2004) | 163 | Preoperative chronic epilepsy: 68/99 (68.7%) seizure-free Preoperative only sporadic seizures: 63/64 (98.4%) seizure-free |
References
19. Churchyard A, Khangure M, Grainger K. Cerebral cavernous angioma. A potentially benign condition? Successful treatment in 16 cases. J Neurol Neurosurg Psychiatry 1992;55:1040–1045
< div class='tao-gold-member'>




