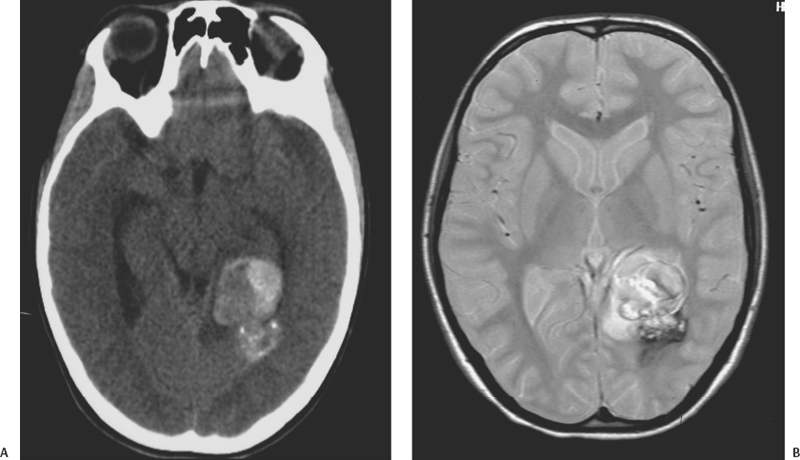
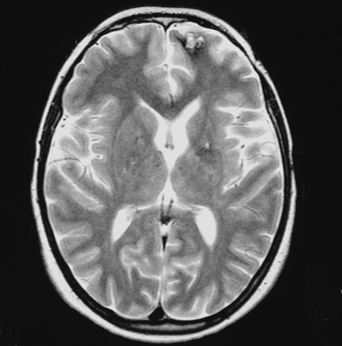
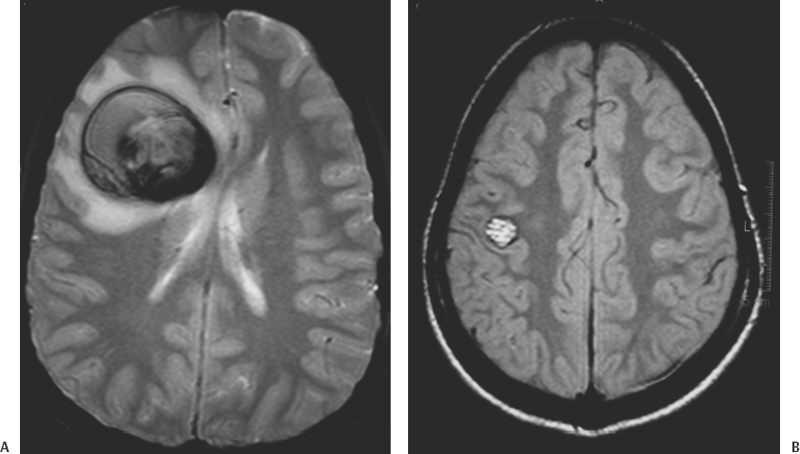
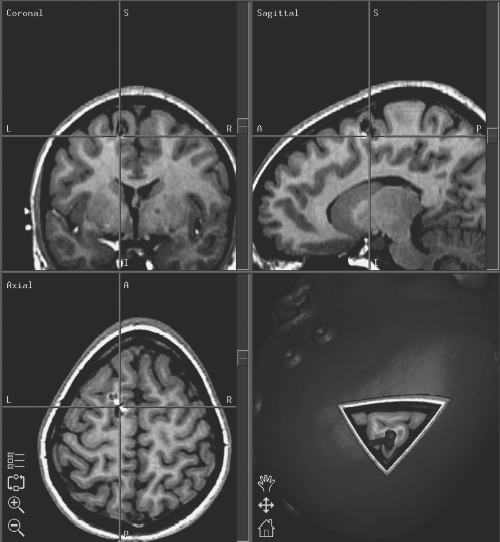
16 Carmine Mottolese, Marc Hermier, Alexandru Szathmari and Carmen Bruno One-fourth of central nervous system cavernous malformations (CMs) occur in the pediatric age group. CMs represent one of the main causes of intracerebral hemorrhage in children, together with ruptured arteriovenous malformations.1,2 Although magnetic resonance imaging (MRI) has improved the diagnosis and understanding of the evolution of CMs in children and adults, the natural history of cerebral CMs is still poorly understood in children. In the pediatric age group, the rate of hemorrhage is higher than in adults, and this fact often justifies a more aggressive surgical approach. This chapter is based on an analysis of the literature and on the experience of the authors.3 The prevalence of CMs is estimated to be between 0.37% and 0.53% in children.4–9 Mazza et al. reported an incidence varying between 1.7% and 18% of all vascular malformations.10 Herter et al. reported a prevalence of 42% of all vascular malformations in the general population, with 25% of CMs found in children.11 Simard et al. reported a pediatric incidence of 23% of all the vascular malformations.12 In the most recent literature, the incidence of pediatric CMs has been reported at about one-fourth of the total number.13,14 Two peaks of incidence are reported: one in early childhood, the other in adolescence.15–18 Our experience3 confirms this age-related distribution, with a peak below 3 years and a second peak after 11 years (age range, 9 months to 17 years). The explanation for this finding remains unclear. The occurrence of cerebral CMs in the prenatal and neonatal period is very low.4,19–22 Gangemi et al. reported on 11 cases from the literature and described 2 additional cases below the age of 1 year.20 We have not observed any case of fetal or neonatal cerebral CMs in our area, but Guibaud et al. reported on three cases of capillary telangiectasia of the cerebellum in fetuses.23 Familial forms of cerebral CMs account for ~20% of pediatric cases.24–26 The pattern of inheritance, in studies dealing with familial forms, is consistent with an autosomal dominant pattern with incomplete clinical penetrance and is sometimes associated with de novo mutations.24 A mutation has been reported on the 7q chromosome in Hispanic Americans but the genetic heterogeneity of inherited cerebral CMs has been demonstrated.27–30 Familial CMs are characterized by a higher incidence of multiple lesions and are more frequent in children.31,32 Systematic screening for multiple CMs has been advocated and is facilitated by MRI. This screening is mandatory because hemorrhagic risk is higher in cases of familial and multiple lesions. In our experience, a familial pattern was found in 15.5% of cases, but we could not demonstrate a higher rate of hemorrhage compared with the sporadic form. A systematic MRI follow-up may also be useful to assess lesion growth from clinically silent hemorrhagic modifications, which may aid in surgical decision-making. Pediatric CMs are generally not associated with other pathologic conditions. There is no need to screen for other visceral locations. In our experience, only one case presented with neurofibromatosis type 1. Another patient presented with a concomitant tectal plate glioma discovered after the surgical removal of a CM responsible for a hemorrhage in the ventricular atrium. In one additional patient, a thalamic CM responsible for a hemorrhage was associated with a giant hemispheric arachnoidal cyst. Cranial irradiation seems to represent a potential risk factor for the development of cerebral CMs. Two cases of cerebral CMs have been observed months after irradiation, performed for a cerebral tumor in one case and a leukemia in another case.33 The development of cerebral CMs after radiotherapy remains controversial, but a growing number of reports sustain this theory. The follow-up of these postirradiation lesions showed growth and hemorrhagic potential as reported by Edwards34 and confirmed by our own experience. The neuropathologic characteristics of pediatric CMs are not different from those in adult patients. CMs are made up of vascular spaces of varying size, lined with a single layer of endothelial cells.35 The blood-filled vascular spaces are separated by collagenous walls of varying thickness that are typically devoid of smooth muscle and elastin, and the histologic features of arteries, veins, or capillaries are usually missing.36–40 The vascular space (caverns) does not demonstrate intervening brain tissue. In children, macroscopic cystic spaces are observed more frequently, presumably due to the higher hemorrhagic rate. The brain around the CM exhibits features of astrocytic gliosis with different thickness and various patterns: a necrotic or an atrophic area with hemosiderin, associated with a zone of cerebral atrophy, calcium, and iron deposits. These perilesional changes could be the substratum for epileptic foci and may be more pronounced when patients are operated on weeks and months after the initial hemorrhage. Collagen fibers arising from the core of CMs with densely proliferating granulation tissue and partially re-endothelialized hemorrhage suggest a possible mechanism for CM growth. In children, and especially before the age of 3 years, microsatellite malformations such as small CMs, capillary telangiectasia, and pseudoangiomatous dysmorphic vessels are found in the surrounding cerebral parenchyma more frequently than in adults.9,39,41–44 This phenomenon led Barrow and Awad to consider an association between various types of vascular malformations and CMs.43–46 The higher risk of hemorrhage could be due to these associated anomalies.43 The association with venous anomalies, frequently reported in adult patients, is less common in children, being reported in only 7.3% of children explored by MRI.4,47–49 We observed this association in only 2 of 47 surgically treated children. The typical cavernous structure can be almost completely obscured by hemorrhage. A capsule is sometimes described with a structure similar to that of a chronic subdural hematoma in giant cystic lesion.50–52 The presence of a capsule can facilitate surgery and can explain the frequent imaging finding of an “encapsulated hematoma.” The location of CMs in children is not very different from that in adult patients. A supratentorial location is more frequent than a posterior fossa location, which accounts for only 20% of cases.53,54 Brain-stem lesions seem more frequent in children than in adults, and the pontine region is the most commonly involved.34,54 CMs are rarely located in the hypothalamic region, the basal ganglia, or the ventricular system. In our experience, the supratentorial location was found in 72% of cases and the frontal region was the most frequently involved, followed by the temporal, the parietal, and the occipital lobes. Spinal CMs are rare in children, and we have observed only one such case. Spinal CMs represent 5 to 12% of intraspinal vascular tumors and 3 to 16% of all vascular malformations.55–59 Follow-up MRI studies have established that CMs can vary in number and size over time, and so they have to be considered as dynamic lesions. In our series, the number of CMs varied at diagnosis from 1 to 12, and small, new lesions were observed during follow-up in three children. MRI is superior to computed tomography (CT) scans for the screening of small lesions. The size of the main lesion is often larger in children, with an average diameter of 6.7 cm, whereas in adults, the average diameter is between 2 mm and 3 cm. In our series, diameters varied from 2 to 3 mm to 11 cm, with an average of 4.5 cm. No clear correlation can be demonstrated between size, risk of bleeding, and late neurologic deficits. According to Awad et al.,60 lesion growth is related to repeated micro-hemorrhages. The extravasation of red cells outside the vessels of the CMs could stimulate an angiogenic factor that is responsible for the formation of coalescent vessels.44,61–63 Larger hemorrhages usually cause acute neurologic symptoms and are frequently responsible for discovery of the lesion in the pediatric age group. The clinical picture of pediatric CMs is variable.18,46,64 They can be asymptomatic or can manifest with acute or progressive clinical symptoms related to hemorrhage, mass effect, or epileptic manifestations. Based on the current literature, it is somewhat difficult to determine the exact proportion of these clinical manifestations in children because hemorrhagic lesions are reported either as responsible for raised intracranial pressure, isolated neurologic focal deficits, and/or seizures. According to Simard et al.,12 the three modes of presentation were equally represented. Seizures, occurring in 25 to 50% of patients, are reported as the primary complaint warranting clinical evaluation.65,66 Vaquero reported epilepsy in 70% of cases, a mass effect presentation in 20% of cases, and a hemorrhagic event in 10% of cases.67 In our experience, seizures frequently are an expression of acute hemorrhage from supratentorial cortical and/or large white matter lesions. The rate of hemorrhage in children is estimated to be between 36% and 78% of symptomatic cases,10,34,63,68,69 whereas in adults, it is between 8% and 37% of cases. We reported a hemorrhagic event in 80% of pediatric cases, and we observed a shorter delay between clinical onset and diagnosis compared with other authors, who reported diagnostic delays up to 20.5 months.34 The higher incidence of hemorrhage in children is responsible for the high rate of acute clinical onset. The typical clinical picture of hemorrhagic CMs located within the brain stem or basal ganglia is associated with coma and focal neurologic deficits. Calcified cerebral CMs can be an incidental finding on skull X-ray views performed for other reasons, such as trauma. Angiography is not useful and should be performed only if there are doubts as to the presence of a true arteriovenous malformation after MRI.70 CMs are no longer “occult” or “cryptic” in the MRI era.2,71–73 CT scans performed in children presenting with acute symptoms usually show a typical hemorrhagic, hyperdense, well-limited lesion with a spherical shape and no or limited surrounding edema. The CM itself is often seen as a smaller area with a different density within the hematoma. CT scans are easy to obtain in emergent situations and are rarely negative in the acute clinical setting. Multiple small lesions, however, are commonly overlooked with CT, unless they are calcified.74–77 MRI is the preferred imaging modality for the diagnosis and follow-up of pediatric CMs. MRI, in addition to the lack of ionizing radiation, is far more sensitive and specific than CT (Figs. 16-1 and 16-2).75,78–80 Gradient echo T2*-weighted sequences are sensitive to magnetic susceptibility artifacts produced by hemoglobin-derived blood products located within and/or around the lesion. Deoxyhemoglobin accounts for the dark signal of acute hematomas on T2*-weighted images, whereas hemosiderin deposits persist after occult or overt hemorrhage. This accounts for the high sensitivity of MRI for the detection of small lesions.3,30,74,81,82 Figure 16-1 A spherical-shaped, well-limited intracerebral hematoma should make one consider the diagnosis of CM, as in this patient with left ventricular CM in the region of the internal wall of the atrium who presented with bleeding. (A) CT scan and (B) MRI. An intracerebral hemorrhagic lesion with a spherical shape and no intraventricular rupture, occurring in an otherwise healthy child or adolescent, should always make one consider the diagnosis of a hemorrhagic cerebral CM (Fig. 16-1).71, 81,83 A hemorrhagic true cerebral arteriovenous malformation (AVM) is the main differential diagnosis at neuroimaging. In this setting, hematoma is more likely to present with an irregular and elongated shape and hemorrhagic extension to the ventricles. We emphasized the usefulness of these morphologic characteristics in a comprehensive imaging classification system.3,84,85 The classic radiologic classification proposed by Zabramski et al. does not demonstrate a strict correlation between CMs and their potential hemorrhagic risk.86 A simple classification that takes into account both imaging and especially surgical features is herein described: Figure 16-2 MRI is more precise than CT scan to display lesions of small volume as in this postirradiation CM of the left frontal region following irradiation of a chiasma-hypothalamic tumor. Figure 16-3 Generally in children, CMs are more frequently responsible for hemorrhage. (A) Encapsulated CM in right frontal lobe with signs of old hemorrhages (type II A1); (B) a lesion in the prerolandic area (type III). In children, huge CMs can be misdiagnosed with CT scans as cerebral tumors such as oligodendrogliomas or ependymomas.49,87 MRI usually allows the correct diagnosis. Cerebral cysticercosis associated with hydrocephalus and epilepsy can be confused with a radiologic picture of multiple CMs. Positive serologic studies and/or parasitic soft tissue calcifications may contribute to the diagnosis of cysticercosis. A focal hyperechoic cerebral mass at antenatal imaging, consistent with a CM complicated by hemorrhage, has been reported in a 23-week-old fetus.88 Fetal capillary telangiectasia is a clinically relevant differential diagnosis because this entity seems to have limited bleeding potential, and should lead to a conservative management of the pregnancy.23 The management strategy for CM in the pediatric population must be carefully considered, keeping in mind the risks of their natural evolution and the risks of their surgical removal.9,16, 36, 63,86,89–93 As suggested in the literature, the management strategy must take into account age, sex, location, and the efficacy of medical treatment to control seizures in epileptic cases. In children, CMs are more frequently symptomatic, and, considering the long life expectancy and the elevated risk of hemorrhage, surgery should be considered regardless of location. The increased risk of further hemorrhage is a strong motive for removal of symptomatic posterior fossa lesions.54,94 A difficult question is the time of surgery. After diagnosis of a hemorrhagic CM, some surgeons prefer to delay lesion removal because this delay might provide a way to obtain a better resection plane around the lesion. Other teams prefer an early surgery because the absence of gliosis could facilitate removal. We think that a delayed surgical procedure is usually safe because the brain is slack, but in many cases the presence of a hematoma is not well tolerated, and thus, early surgery is necessary to reduce intracranial hypertension. As for other neurosurgical entities, intracranial hypertension in children can have a rapid evolution with an increased risk of mortality. When located in the brain stem, the surgical removal of a CM is a challenge, especially when located far from the ependymal plane.95,96 Some authors have emphasized the possible entry zone avoiding the anatomic localization of the nuclei of the cranial nerves and of the reticular system.97,98 The inferior losange of the floor of the fourth ventricle is particularly rich in sensory and motor nuclei. When located in the bulbar and the pontine region, the surgical approach has to safeguard these nuclei to avoid irreversible deficits of deglutition, phonation, and taste. In children with repeated seizures despite medical treatment, surgery plays an important role to cure epilepsy definitively. The indication for surgical removal of CMs with epilepsy should be discussed for each case and requires a thorough evaluation with long-duration electroencephalogram (EEG) recording and video EEG. Invasive investigations may be mandatory to assess the true localization of the epileptic focus, which can sometimes be distant from the CM, especially in the case of mesial lobe epilepsy.99 A point of surgical controversy is the removal of the hemosiderin capsule located around the lesion that can represent an irritating element.100 The epileptogenicity of these lesions has been credited to the ongoing deposition of iron and blood breakdown product in the periphery of the lesion as reported by Maraire and Awald.63 The management of CMs presenting with seizures is detailed elsewhere in this book. The difficulty in differentiating lesion from compressed or atrophic tissue55 leads some surgeons to remove only the CM. Removing the surrounding brain tissue may also generate a deleterious vasogenic reaction, which can increase the risks of sequelae, especially if the lesion is located near a functional area. We think that it is sufficient to remove only the CM, sparing the resection of the parenchyma around the lesion. In case of multiple CMs, only bleeding or symptomatic lesions should be removed and only if the lesions show imaging evidence of fresh hemorrhage or lesion growth. If the localization spares functional areas, we recommend systematic removal. Technically, the removal of CMs in children is not different from that in adults, but, in children, particular care must be exercised, especially in very young patients, to avoid significant blood loss. The complete removal of the lesion is the main goal of surgery, because, as reported by Scott et al.,21,22 residual lesions are associated with a high incidence of hemorrhage and a high risk of neurologic deficits. We agree with other studies that indicate that if the removal is not total, the residual nodule should be the subject of a second-look surgery.54 In children, basal approaches involving orbito-zygomatic osteotomies, as used in adult patients, should be avoided, especially in children younger than 5 years. The use of neuronavigation (in children more than 3 years of age) allows the best trajectory, as well as easy localization for small and deeply located lesions (Fig. 16-4). Because of the use of neuronavigation, we are able to make a small linear skin incision and a small spherical-shaped bone flap located at the level of the projection of the lesion on the skull. When the lesion is located in a functional area, we try to achieve a trajectory that will avoid severe deficits. Thus, surgery through the cerebral sulcus is possible to reach the lesion, sparing the removal of the cerebral parenchyma. Finally, the use of neuronavigation has rendered obsolete stereotactic procedures for localizing the lesion. If a cortectomy is necessary because the lesion is deeply located, the entry point can be established to avoid functional areas. Cortical stimulation can help the surgeon when the lesions are located in or near the motor area (Fig. 16-5).101 Surgery under local anesthesia is difficult in children, and so lesions located in the language area (frequently an indication to perform surgery under local anesthesia in adults) have to be removed using general anesthesia. In this critical location, in older children, the fusion of morphologic and functional MRI data, coupled with neuronavigation, can be useful for the surgical removal; however, the interpretation of pediatric functional MRI can be problematic.102 Sparing arteries and veins is particularly important in functional regions. The use of microscopic techniques has improved the results and decreased the incidence of complications after surgery in such critical areas. In our opinion, it is better to remain outside the lesion to ensure complete removal. If the surgeon invades the lesion, there is a small risk of an uncontrollable hemorrhage and it can be difficult to find an adequate plane to remove the CM completely. We usually do not remove the lesion using a piecemeal technique or by using the ultrasonic aspirator. The use of a cottonoid to push the lesion slightly allows visibility of the small afferent arteries and allows following of the yellow-reddish plane that is indicative of the true limits of the lesion. The microscope also allows visualization of satellite veins that must be spared to avoid cerebral infarcts. We do not think that a staged surgical procedure is useful for giant lesions, and we do not use the laser beam for the removal of such lesions.
Cavernous Malformations in Children and Adolescents
 Epidemiology
Epidemiology
Incidence
Familial Cerebral Cavernomas
Associated Diseases
Neuropathology
Location
Lesion Number, Size, and Growth
 Clinical Presentation
Clinical Presentation
 Imaging Findings
Imaging Findings
 Differential Diagnosis
Differential Diagnosis
 Treatment
Treatment
Surgical Treatment
Cavernous Malformations in Children and Adolescents
Only gold members can continue reading. Log In or Register to continue

Full access? Get Clinical Tree




