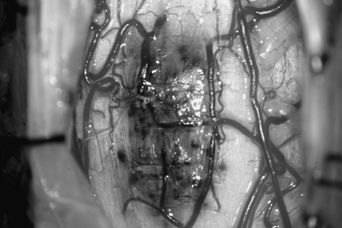
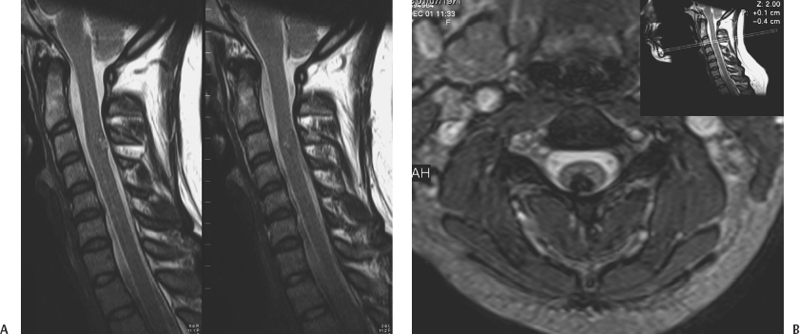
12 Paolo Perrini, Uygur Er, Robert F. Spetzler and Giuseppe Lanzino Cavernous malformations (CMs) of the spinal cord had been considered to be extremely rare until the widespread availability of magnetic resonance imaging (MRI) in the 1980s. Spinal cord CMs were first described in the early 1900s.1 In 1912, Schultze reported the first successful surgical removal of a cervical CM in a 29-year-old-man. After surgical resection, the patient experienced improvement of his neurologic function.2 In a literature review conducted in 1929, Globus and Doshay3 reported this case along with two additional others. Until the mid-1980s, only a few additional anecdotal cases were reported for a total account of 19 cases recorded until 1985. Because of the widespread use of MRI, spinal cord CMs have been disclosed with increasing frequency, and in 1988, Cosgrove et al.4 and McCormick et al.5 reported surgical series of five and six patients, respectively. Since then, several additional series have been reported, and intramedullary CMs have become a recognized potential cause of myelopathy especially in young individuals. CMs affecting the spine and spinal cord display a wide spectrum of pathologic findings to include lesions involving the vertebral body and spinal extramedullary lesions of the intra-and extradural spaces. This chapter, however, focuses only on intramedullary CMs. CMs account for 5 to 12% of spinal vascular malformations5,6 and represent 3 to 5% of all spine lesions in surgical series or autopsy studies. Some authors have reported a slight female preponderance although others have failed to show this.6–8 More commonly, spinal cord CMs present in the fourth decade, although the age range in the literature varies between 129 to 881 years. Approximately 10% of all intramedullary spinal cord CMs are observed in the pediatric population.10 Pediatric patients do not show a gender imbalance and usually present with a more acute episode compared with adults.10,11 CMs can occur anywhere along the spinal cord but appear to have a predilection for the thoracic levels followed by the cervical cord. Lesions involving the conus medullaris or cauda equina are less common.12 Numerous reports suggest that patients with spinal cord CMs are at an increased risk for multiple neuraxis CMs.13–15 Vishteh et al.15 reported multiple CMs in almost half of 17 patients with symptomatic intramedullary CMs who had both spinal and brain imaging. On the basis of this data, it is suggested that patients with spinal cord CMs should have a careful family history and screening MRI of the brain. Angiomas of the skin and others organs such as kidney and liver have also been reported in association with intramedullary CMs.4,5,16,17 CMs present similar gross and histopathologic features whether they involve the brain or spinal cord. The macroscopic features of spinal cord CMs are similar in each case, showing a dark blue-brown mulberry- or raspberry-shaped lesion with surrounding gliosis and hemosiderin staining. The lesions are well circumscribed, but “tongues” of CMs often infiltrate the surrounding gliotic plane. The size of these lesions varies from a few millimeters to centimeters. Due to the slow progressive growth of the malformation, the spinal cord can be completely replaced by the CM without visible enlargement. The cord surface usually presents a typical area of bluish discoloration (Fig. 12-1), but in case of small lesions the spinal surface can present normal appearance. The surrounding parenchymal tissue often shows evidence of repeated hemorrhage with deposition of hemosiderin in the gliotic tissue. Calcification is rare and sometimes can be present as small flecks of bone and focal ossification.18,19 Cosgrove et al.4 observed ossification in one of five cases of intramedullary cavernous angiomas, and Ogilvy et al.18 reported flecks of calcification and focal ossification in only one case of their series. Naim-Ur-Rahman et al.20 reported a large (2 × 3 cm) cavitating intramedullary thoracic CM with laminated shell-like pattern of calcification. In this case, the heavily calcified outer shell with spiky projections into the surrounding parenchyma precluded total removal. De novo spontaneous occurrence of spinal cord CMs has been documented.21 Appearance of spinal cord CMs after radiation22,23 has also been observed. In the two reported cases of intramedullary spinal CMs after spinal irradiation,22,23 the latency period was 5 and 13 years. Figure 12-1 Intraoperative microscopic view of a cervical cord CM. The malformation was immediately evident after exposure of the dorsal surface of the cord through a posterior cervical laminoplasty. Under high magnification, the characteristic bluish discoloration of the CM is noted. Brownish discoloration of the cord surface adjacent to the CM is visible. This brownish discoloration is caused by hemosiderin deposition around the CM. (From Lemole GM, Lanzino G, Henn JS, Spetzler RF. Spinal cord cavernous malformations. Operative Techniques in Neurosurgery 2002;5:155–160. Reprinted with permission.) The clinical presentation of spinal cord CMs is variable. In the past, the intermittent/remittent clinical course, often in young individuals, led to the wrong diagnosis of a de-myelinating process or Foix-Alajouanine syndrome.4 Although the clinical course is unpredictable, the onset of symptoms usually falls into one of three patterns: acute, progressive, or episodic.4,5,7,12 Acute onset of neurologic symptoms is caused by hemorrhage within or around the lesion. This is supported in surgical cases by the intraoperative findings of recent hematoma within the cord.5,18,24,25 Patients can present with motor and sensory deficits as well as with bowel and bladder dysfunction. Pain is present in one-half of the patients and usually corresponds with the level of the CM.26 Although precipitating factors such as trauma, pregnancy, and strenuous activity have been reported, it is unclear whether these associations are coincidental or causative.5,27 Subarachnoid hemorrhage can occur particularly with lesions of the cauda equina28,29 and in CMs located on the spinal cord surface.30 CMs of the cauda are adherent to one or more nerve roots.31 A pattern of slow progressive decline in neurologic function has been described in 41% of patients with intramedullary CMs.12 Various mechanisms can induce this pattern of clinical worsening. Some authors suggested enlargement of the CM due to repeated small hemorrhages or gradual thrombosis.18 Neurotoxic effect of hemosiderin and compromise of the surrounding microcirculation have been proposed as a possible cause to explain progressive myelopathy.5 CMs are low-flow shunts, and hemodynamic alterations, such as arterial steal or venous hypertension, are unlikely to play a role in the progressive deterioration. Episodic and stepwise deterioration have been described in 30% of patients with spinal cord CMs.12 This pattern can mimic other clinical conditions such as transverse myelitis, multiple sclerosis, and other progressive demyelinating diseases. In these cases, patients present with multiple episodes of neurologic worsening with gradual but usually only partial improvement between events. Multiple hemorrhages from the lesion over several years with subsequent gradual neurologic recovery can explain this clinical pattern. The presence of hemosiderin in the surrounding tissue and blood products of various ages within the lesion is a common surgical finding in such patients. Hydrocephalus with increased cerebrospinal fluid protein content has been described in a patient with a cavernoma of the cauda equina,32 probably resulting from subclinical spillage of blood in the subarachnoid space. With the advent of MRI, incidental asymptomatic lesions are encountered, and at this point their natural history is unknown.16,17 In symptomatic lesions, Zevgaridis et al.12 estimated an average bleeding rate of 1.4% per lesion per year. This figure, however, was obtained assuming that patients were harboring spinal CMs since birth, but several reports suggest that these lesions can be acquired later in life.21,22,27 Symptoms can be present for many years before a correct diagnosis is made. From a pooling of literature data, the overall median duration of pretreatment symptoms is 32 months.12 The interval between presentation and diagnosis is shorter in more recent series as MRI is currently performed early after initial presentation. As previously mentioned, a history of multiple episodes of stepwise deterioration, slow progression of neurologic signs, or sudden onset of neurologic deficits especially in a young individual should raise the suspicion of a spinal cord CM. Nevertheless, spinal cord inflammatory disease such as multiple sclerosis and transverse myelitis should be excluded. Computed tomography and myelography have been used in the past for the diagnosis of CMs of the spinal cord but have low sensitivity and are of historical significance. These studies may show evidence of spinal cord widening, suggesting an intramedullary lesion.4,33,34 Axial computed tomographic scans may also show presence of acute hemorrhage and calcification. MRI is the procedure of choice in the diagnosis of spinal cord CMs. The MRI (Fig. 12-2) characteristics of spinal cord CMs are no different than intracranial CMs35 and are treated in detail elsewhere in this book. There is usually little if any enhancement on T1-weighted images with gadolinium. T2-weighted images can demonstrate increased signal surrounding the lesion for the presence of the edema as well as a hypointense ring of hemosiderin in the gliotic plane around the malformation. Sometimes in cases of small lesions, spinal MRI can be misleading for surgical planning when trying to estimate where the malformation comes closest to the surface of the spinal cord. Malformation thought on MRI to be located on the surface of the spinal cord can be found deeper only after a myelotomy. Vishteh et al.36 reported that MRI had a 17.6% false-positive rate in estimating whether a spinal cord CM reaches the pial surface. Spinal angiography is of little value because cavernous angiomas are angiographically occult, although an associated “tumor blush” has been described.35,37,38 In some cases, however, spinal angiography is warranted to rule out the presence of a spinal cord arteriovenous malformation because the MRI characteristics of spinal cord CMs are not always clear-cut, especially in small lesions.
Cavernous Malformations of the Spinal Cord
 Epidemiology
Epidemiology
 Pathology
Pathology
 Clinical Presentation
Clinical Presentation
 Diagnosis and Indications for Treatment
Diagnosis and Indications for Treatment
Cavernous Malformations of the Spinal Cord
Only gold members can continue reading. Log In or Register to continue

Full access? Get Clinical Tree




