CHAPTER 34 Cavernous Sinus Meningiomas
HISTORY
It is widely cited that Galen had performed dissections in lower vertebrates, described that the internal carotid artery formed a vascular rete in the neck, and transferred this finding to his teachings on the human body.1 As Galen’s teachings formed the backbone of medicine during this time, his description was not challenged. A clearer understanding of the anatomy of the parasellar area began to emerge after the former dogma was challenged in the second half of the 17th century. In 1658 Wepfer described that the carotid artery passed through a deep and conspicuous sinus during its course through the skull base. In 1685 Vieussens described the relation of cranial nerves to the outer wall of the sinus. These initial references were followed by Ridley’s first detailed description of the CS in 1695. Winslow, however, was the first to use the term “cavernous sinus” in 1734, as an analogy to the corpus cavernosum of the penis. Winslow clearly described the anatomy including the placement of the internal carotid artery and the IIIrd, IVth, Vth, and VIth cranial nerves within the sinus. The medical literature contains only very few reports from the period between the 18th and the second half of the 20th centuries. These sporadic reports include the first tumor removal from the CS, performed by Krogius in 1896. Browder2 performed the first successful surgery in 1937 for a caroticocavernous fistula. Although the state of the art in treatment of caroticocavernous fistulas at that time was carotid ligation, Browder had surgically explored the sinus and electrocoagulated the fistula. Despite such single achievements, the approach to the CS was outside the scope of neurosurgery. The dogma of “no man’s land” was challenged first by Parkinson. The first direct and systematic approach to the CS was performed by Parkinson in 1965.3 This operation was performed again for a high-flow caroticocavernous fistula, using hypothermia and extracorporeal circulation. The initial report drew attention to the so-called “anatomic jewel box,” and systematic studies on the microsurgical anatomy by Taptas,4 Dolenc,5 Parkinson,3 and Umansky and Nathan6 were followed by others. A better appreciation of the microsurgical anatomy led to a description of novel surgical approaches. Hakuba7 described the combined orbitozygomatic infratemporal epidural and subdural approach in 1982. This was followed by the very popular frontotemporal epidural approach of Dolenc in 1983.8 Sekhar9 reported the preauricular infratemporal approach in 1986 and Al-Mefty10 reported the cranio-orbitozygomatic and extended middle fossa zygomatic approaches in 1988, which led to the popularization of CS surgery. The start of the skull-base era marked the appearance of reports describing very high total resection rates and consistent decreases in mortality. However, the initial enthusiasm in the computed tomography (CT) era of 1980s was soon followed by the demonstration of a high incidence of tumor residuals as detected with the more sensitive magnetic resonance imaging (MRI) technology. Today we know more of the biology of CS meningiomas. Accumulating expertise has taught us that total resection of CS meningiomas is possible but that it can rarely be achieved for meningiomas that involve the CS proper. Seen from an oncologic perspective, such total resections can only be palliative, considering the high incidence of local invasion of meningiomas. In addition, we now know that CS exploration is associated with significant cranial nerve morbidity.
SURGICAL ANATOMY
The CS is a parasellar anatomic compartment that contains bilateral internal carotid arteries, the IIIrd, IVth, first two divisions of the Vth and VIth cranial nerves, the pericarotid sympathetic plexus, and a network of interconnected venous spaces.5 This anatomic compartment is located at both sides of the sellar space, at the junction of the anterior and middle cranial fossae. Anterolaterally it neighbors the sphenoid ridge and posterolaterally the petroclival ridge. The space is limited on all sides by dura mater. The inferior and medial walls are formed as a continuum of the periosteal layer of the sellar dura. The superior and lateral walls are formed by fusion of the dural sheaths of IIIrd, IVth, and first divisions of the Vth cranial nerve in the embryologic period. In the adult, the lateral wall of the CS is composed of two layers, and invasion by laterally situated meningiomas may involve the lateral or the medial cavernous component. The CS is a meeting point for the intracranial venous circulation. This pair of venous sinuses on either side of the sella is connected by intercavernous sinuses and receives blood from the superior and inferior ophthalmic veins; it connects posteriorly to the petroclival venous plexus, to the sigmoid sinus via the superior petrosal sinus, to the jugular bulb through the inferior petrosal sinus, and to the venous sinuses along the sphenoid wing and is connected with the deep facial veins through the pterygoid venous plexus. Within the CS, the internal carotid artery (ICA) is the most medial structure and sits against the carotid sulcus of the sphenoid bone. Laterally the ICA neighbors the VIth cranial nerve, which is the only cranial nerve to course within the “cavernous sinus proper.” The ICA enters the CS as it pierces through the sinus membrane, by the foramen lacerum. The foramen lacerum contains a fibrocartilaginous tissue in adult and the ICA courses tangential to this. At this point the “lacerum segment” of the ICA is covered by the “inferior sphenopetrosal ligament,” which was first described by Lang and Strobel. The authors described two parts: the pars sagittalis (which is the petrolingual ligament spanning from the petrous apex to the lingual sphenoidale) and the pars transversalis (which is formed by the endosteal dura of the superior aspect of the petrosphenoidal suture). Both of these structures blend into a fibrous ring circumferentially surrounding the ICA. Sekhar has stated that this ring is formed by dural fibers from the carotid canal. Dolenc has called this structure “the lateral ring.” In this region the ICA is neighbored laterally by the greater superficial petrosal nerve, which exits the foramen lacerum and enters the pterygoid canal. The artery forms a siphon within the CS by assuming an anterosuperior bend posteriorly and a superomedial bend anteriorly before it leaves the CS. At its exit from the cavernus sinus, the ICA pierces through the carotico-oculomotor membrane and travels for a short segment, extradurally under the anterior clinoid process, before it becomes intradural again. The two dural rings are called the proximal and distal dural rings and are a continuum of the roof of the CS, which in turn is formed as a continuum of the tentorial dura. The superior wall sends two reflections to the anterior clinoid process forming the two membranes. The extradural segment of the ICA behind the ACP was named the “clinoid segment” by Bouthilier and colleagues, “the siphon segment” by Fukushima and Day, or is simply the Dolenc’s space.11 The proximal dural ring is formed by the medial and inferior periosteoum of the ACP and only partially surrounds the ICA. Although the clinoid segment is extracevernous, venous plexi may continue into this incomplete dural ring. The distal dural ring, which was first described by Perneczky in 1985, surrounds the ICA circumferentially and it blends laterally into the adventitia of the ICA, the roof of the CS, and the dural fold known as the falciform ligament.12 The tough fibrous coverings at the entry and exit of the ICA to the CS are called dural rings and retain the bends of the ICA siphon under high pulsatile pressures. Within the CS, the ICA gives off three main branches. The posterior or the meningohypohyseal truncus leaves the posterior genu at its superior surface. The distal branches of this truncus are the tentorial artery of Bernasconi and Cassinari, the dorsal meningeal artery, and the inferior hypophyseal arteries. At the midpoint of the horizontal segment the ICA gives off the lateral main stem or the inferior CS artery at its lateral side. This stem forms an important source of anastomosis with the external carotid artery (ECA) via the arteria meningea media at the foramen spinosum, the maxillary artery at the foramen rotundum, and at the foramen ovale. The third and last branch of the cavernous ICA is McConnell’s capsullary artery, which supplies the capsule of the pituitary gland.
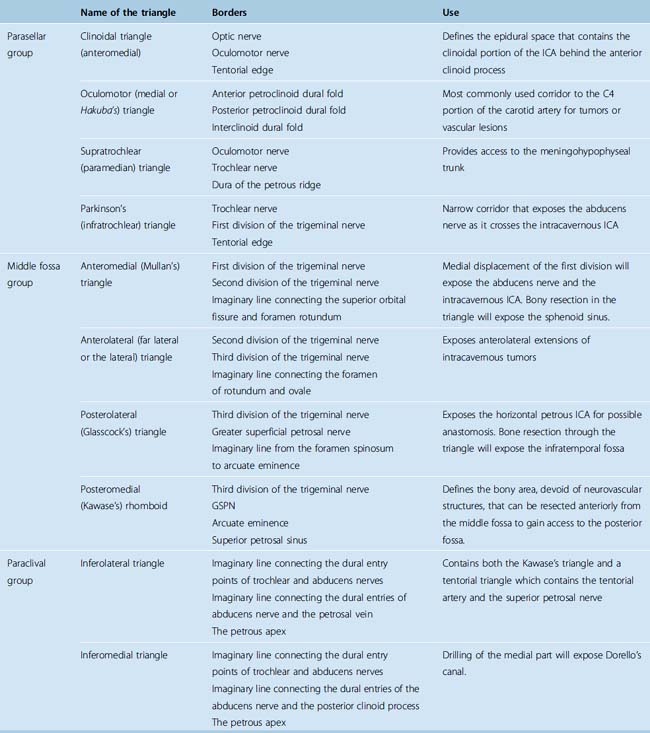
The entry to the CS is accomplished through multiple surgical corridors. Various such corridors have been described by pioneering surgeons. Four of these are the most commonly used and versatile; however, a much more extensive, systematic description has been provided by Fukushima.13 A description of the triangles is provided in Table 34-1; however, several of the triangles deserve special consideration. Two of the four most commonly used triangles are on the roof of the CS, which are the clinoidal and occulomotor triangles. The lateral wall bears the supratrochlear and the infratrochlear (Parkinson’s) triangles. The supratrochlear or the superior triangle is formed by the oculomotor and the trochlear nerves and the posterior margin is formed by dura along the petrous ridge. The supratrochlear triangle provides an exposure of the meningohypohyseal trunk. The infratrochlear or the lateral triangle was first described by Parkinson in 1965. It is bound by the trochlear and the first division of the trigeminal nerves. The dura of the petrous ridge forms the posterior margin. The medial triangle is used to access the cavernous ICA for a direct approach to intracarotid aneurysms and most CS neoplasms. It is delimited by the intradural carotid artery, the posterior clinoid process, the porus oculomotorus, and the siphon angle of the carotid artery. Two additional triangles describe important entry routes through the petrous bone. The posterolateral triangle was first described by Glasscock in 1968 to define the horizontal intrapetrous ICA. The exposure at this triangle is used to gain proximal control of the ICA in an intracavernous procedure. The posteromedial triangle was described by Kawase to represent the anterior petrous projection of the bone that has to be drilled from the petrous apex to gain access to the posterior fossa without encountering neurovascular structures within the bone.
PATHOLOGY
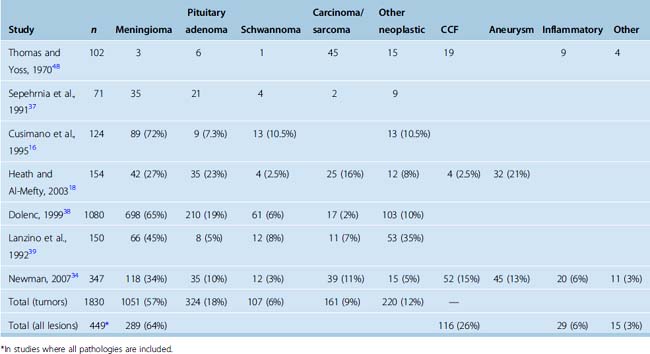
The reported relative incidences of different CS pathologies have varied considerably in the literature, mostly due differences in study methodology. Earlier studies focused on symptomatology and reported high incidences of CS syndrome due to trauma and iatrogenic injuries. With advances in diagnostic radiology, the spectrum of lesions became more clearly defined. The three most common space-occupying lesions in the CS are tumors, vascular lesions, and infectious/inflammatory lesions. Again, most clinical series are biased by their institutional referral patterns. A summary of large studies is provided in Table 34-2, which shows that roughly two thirds of CS masses are tumors and roughly one fourth are vascular pathologies. Among neoplastic lesions, meningioma is the most commonly reported pathology in most modern series and makes up roughly two thirds of all tumors. Invasive pituitary adenomas and schwannomas (of the trigeminal nerve and other cranial and noncranial nerves) are also commonly encountered. A list of reported pathologies is provided in Table 34-3. The surgical results in malignant pathologies are not satisfactory, and surgery is seldom indicated in such cases, mostly to establish a tissue diagnosis where radiologic studies are indecisive. The role of surgery and its extent is the source of another debate and is discussed below. Surgical results in invasive pituitary adenomas are poor.14 In fact, several authors have reported that the total resection rate for invasive pituitary adenomas is the lowest among all benign nonmeningeal tumors of the CS. Conversely, the surgical results for schwannomas and neurinomas are better than in meningiomas. Cavernous hemangiomas of the CS can also be resected with good surgical results, if an intracapsular resection can be achieved. Surgery remains the best treatment option for chordomas and chondrosarcomas; however, the CS portion is difficult to resect totally, more so in chordomas.
TABLE 34-3 Differential diagnosis of cavernous sinus masses
| Tumors | |
| Vascular lesions | |
| Infectious and inflammatorylesions |
INCIDENCE AND CLINICAL MANIFESTATIONS
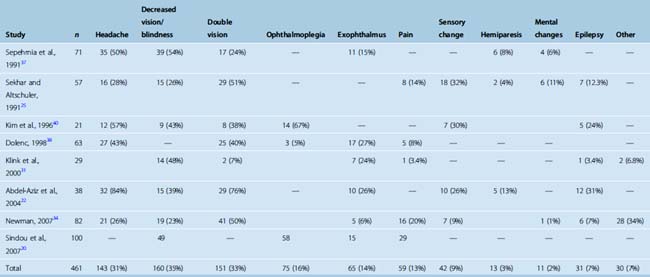
The most common presenting symptom of a CS meningioma is decreased vision in the ipsilateral eye. The incidence of this symptom varies between 23% and 54% among studies (Table 34-4). Headaches are present in roughly one third of patients. A classical finding in CS meningiomas is disturbance of ocular movements. Proptosis and varying degrees of ocular movement limitations ranging from complete ophthalmoplegia to slight functional disturbances can be observed. Trigeminal involvement resulting in facial pain syndromes or sensory changes is observed in only a minority of patients. Other findings such as hemiparesis, mental changes, or epilepsy are observed in even a smaller minority. Incidental CS meningiomas are commonly found during cranial radiologic studies. Almost none of the clinical studies on treatment of CS meningiomas report the incidence of asymptomatic cases. Nakamura and colleagues15 reported that CS meningiomas made up 17% of 41 incidentally found meningiomas. A quick glance at the published series indicates that the symptomatology differs slightly between meningiomas and nonmeningeal tumors of the CS. The incidences of headache and double vision are higher in nonmeningial tumors of the CS.
DIAGNOSTIC STUDIES
As CT and MRI provide a significant amount of information both on the pathoanatomy of the lesion and its effects on surrounding neurovascular structures, angiography is not universally indicated. However, if there is a significant risk of internal carotid involvement, digital subtraction angiography (DSA) can provide very valuable functional information. First, DSA can provide supplementary diagnostic information on the carotid involvement by demonstrating occlusion, narrowing, luminal changes, and pseudoaneurysm formation. Second, using cross compression, the DSA will provide information on the vascular reserve of the contralateral ICA and the posterior circulation. Rupture of the internal carotid artery may occur during CS exploration, which may necessitate a carotid takedown. A postoperative major disabling stroke is reported in roughly 5% of cases with CS meningiomas.16–18 In patients with a significant risk of internal carotid involvement, a clearer and more objective picture on the vascular reserve can be provided with a balloon occlusion test. The test consists of angiographic catheterization of the internal carotid artery and occlusion of the internal carotid artery with an intraluminal balloon with constant neurologic monitoring. The development of neurologic symptomatology on closure of the ICA indicates that the contralateral ICA and the posterior circulation cannot provide adequate perfusion compensation, which will significantly affect the surgical strategy. The balloon occlusion test can also be combined with single-photon emission computed tomography (SPECT) imaging or xenon blood flow studies to increase its reliability. Patients are ultimately stratified to groups of low-intermediate and high risk of neurologic complications after a possible carotid takedown.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree