17 | Central Cord Syndrome |
 | Case Presentation |
History and Physical Examination
A 55-year-old woman presented to the emergency room after tripping forward in an elevator, striking her forehead, and suffering immediate and complete upper and lower extremity paralysis. She remained alert and oriented throughout, and by the time she was brought to the hospital she had regained some voluntary movement in her arms and legs. Examination 1 hour after the fall revealed a bruise on her forehead, and a diffusely tender posterior cervical spine with no step deformity. Her neurological examination revealed full strength in the elbow flexors (grade 5/5) but diminishing strength in wrist extensors (grade 3/5), elbow extensors (grade 3/5), finger flexors (grade 2/5) and finger abductors (grade 1/5). She had normal strength in the lower extremity myotomes, giving her an American Spinal Injury Association (ASIA) motor score of 78. Her sensation was diminished to light touch and to pinprick from the C6 to T1 dermatomes but was otherwise normal. Plantar responses were upgoing, and the bulbo-cavernosus reflex was present.
Radiological Findings
Initial radiographic screening in the emergency room consisted of anteroposterior and lateral x-rays of the cervical spine (Fig. 17–1A). No acute fractures or signs of a ligamentous injury were seen, but degenerative changes were noted at C3-4, C4-5, and C5-6. The cervical spine was noted to be lordotic. A computed tomographic (CT) scan confirmed that there were no osseous abnormalities and that the alignment was normal (Fig. 17–1B). Subsequent magnetic resonance imaging (MRI) confirmed the absence of an acute disk or ligamentous injury but revealed the presence of diffuse degenerative spondylosis causing severe stenosis within a congenitally narrow spinal canal (Fig. 17–1C). There was obvious signal change within the spinal cord extending from the C3-4 disk level to the mid-C6 level, in keeping with an acute spinal cord injury.
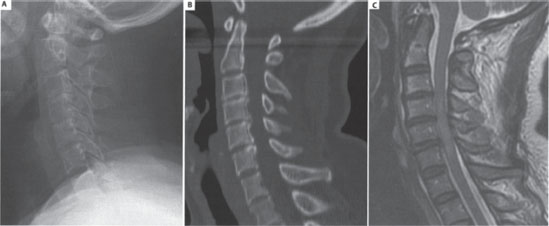
Figure 17–1 Preoperative imaging of the cervical spine. Note the maintenance of lordosis on (A) the lateral radiograph and the absence of any fractures, subluxations, or abnormal soft-tissue swelling. (B) The sagittal reconstructed computed tomographic scan confirms the absence of bony injury, and makes evident the congenital stenosis of the cervical spinal canal. (C) T2-weighted sagittal magnetic resonance imaging demonstrates the canal stenosis to be marked at the C3-4, C4-5, and C5-6 levels, and illustrates the spinal cord injury, with signal change extending within the cord at these levels.
Diagnosis
The neurological findings of disproportionately greater impairment of upper versus lower extremity function are consistent with a central cord syndrome of incomplete spinal cord injury. Many features of this patient’s presentation were characteristic of central cord injuries: the low energy fall, the hyperextension mechanism of injury, the absence of acute osteoligamentous injury, the degenerative changes superimposed upon a congenitally stenotic spinal canal, and the signal change within the spinal cord.
 | Background |
The first descriptive characterization of the acute, traumatic central cord syndrome was initially published in 1954 by Schneider and colleagues as a pattern of incomplete paralysis characterized primarily by a disproportionate motor impairment of the upper limbs compared with the lower limbs.1 Associated with this are varying degrees of sensory disturbance and bladder dysfunction. This pattern of incomplete tetraplegia most frequently occurs in elderly individuals with congenital or spondylotic stenosis of the cervical spinal canal who suffer a hyperextension injury of the neck. This low-energy mechanism of injury typically does not cause an acute fracture or dislocation that would make the spine unstable, but the cord is compressed in the anteroposterior dimension by the infolding of the ligamentum flavum and lamina, which can narrow the canal diameter by as much as 30%.2 Although this is the most common clinical presentation, individuals of any age in whom the cervical spinal canal is abruptly narrowed secondary to an unstable injury or an acute disk herniation can sustain a central cord syndrome of incomplete tetraplegia. This point is important to recognize so that one does not overlook an unstable cervical column injury in a patient with the neurological findings of a central cord syndrome. The central cord pattern of paralysis is the most commonly observed pattern of incomplete spinal cord injury.
In 1954, when Schneider et al’s original description of the central cord syndrome was published, it was widely believed that the corticospinal tract was somatotopically organized, with the axons subserving the sacral, lumbar, thoracic, and cervical regions positioned in a lateral to medial fashion. Although it had been previously postulated that a centrally developing hematomyelia affected these medially placed cervical fibers and thus accounted for a disproportionate amount of upper extremity weakness, Schneider felt that transient swelling or edema within the central parenchyma was the more likely pathology, given that most patients eventually recovered on their own. He therefore coined the term acute central cervical cord injury to be more inclusive of the proposed neuropathology of this clinical syndrome. More than 3 decades later, the concept of a central hematomyelia in patients with central cord injuries was further refuted by neuroradiological and pathological studies done by Quencer et al.3
Furthermore, the existence of such a somatotopic organization of the corticospinal tract, with the axons subserving upper extremity function running medially to those subserving lower extremity function, has never been established in anatomical studies (despite the fact that this has been propagated through textbooks for many years). Recent neuroanatomical studies of patients who sustain a central cord injury suggest that the loss of upper extremity function observed in the central cord syndrome is still related to injury of the large, rapidly conducting myelinated fibers of the lateral corticospinal tract.4 However, rather than being somatotopically organized, the basis for the predominant upper extremity impairment relates to the fact that the main function of the corticospinal tract is to mediate fine motor function of the arm, and of the hand in particular (elegantly reviewed by Levi and colleagues5).
In general, patients who present with an incomplete spinal cord injury have a far better prognosis for neurological recovery than patients who sustain a complete spinal cord injury. Consistent with this, the natural history for patients with an acute central cord syndrome is that they will typically experience a substantial improvement in their neurological function, although after neurologically plateauing they may suffer a late deterioration in function related to spasticity and pain.6,7 Historically, a nonoperative approach was widely accepted after being initially advocated by Schneider et al, who reported that the majority of patients sustained some spontaneous neurological improvement without surgical intervention.1 Surgical management at the time of Schneider et al’s initial description of this syndrome included a laminectomy, durotomy, anterior exploration of the cord, and dentate ligament sectioning. To their credit, Schneider et al recognized that the conservative approach yielded a better outcome, and for decades this was adhered to. In 1971, however, Bosch and colleagues were the first to shed light on the limitations of this approach.6 Their review of 42 conservatively treated patients with central cord syndrome revealed that, although most experienced some objective neurological improvement, approximately half were still extremely disabled with respect to hand function, ambulation, and bowel/bladder control. The results of performing more contemporary anterior and posterior extradural approaches to decompressing the spinal cord in patients with central cord injuries were then reported in the early 1980s by Brodkey et al and Bose et al, with both groups having observed significantly improved neurological recovery in their surgically managed patients.8,9 Since then, many authors have reported the neurological benefits of surgical decompression in this population of patients.
Despite this, much has yet to be answered about the optimal management of patients with acute central cord syndrome.10 Given that patients who sustain an acute central cord injury can be of a wide age spectrum, can present with a wide variety of cervical pathology, and can suffer very different severities of neurological injury, defining the optimal treatment has been extremely difficult. Complicating this is the fact that most patients with this pattern of incomplete tetraplegia and a stable cervical spine will experience objective neurological improvement over time without any surgical intervention. As such, many aspects of the management of acute central cord syndrome remain controversial. Furthermore, given that these patients suffer a combination of motor, sensory, and bowel/bladder dysfunction, it is difficult to even know what the most appropriate outcome measure is to quantify their functional recovery. Our institution recently described the functional status and health-related quality of life for a cohort of surgically and conservatively treated patients with central cord syndromes.11 At a minimum of 2 years postinjury, we observed that, although the patients achieved a significant improvement in their ASIA motor scores, persistent spasticity often precluded useful function. In particular, patients who experienced the greatest recovery of motor function also suffered the greatest spasticity, which limited them from achieving the functional benefits that one would expect them to enjoy given their high motor scores. Clearly, this is an area in need of further study, not just in central cord injury but in other syndromes of complete or incomplete spinal cord injury as well.
Having said that, in the patient who presents with an unstable injury of the cervical spinal column, there is a compelling rationale to stabilize the spine, at which time a concomitant decompression can be performed. The real controversy arises in patients such as the one presented in this chapter, in which there is no evidence of an unstable cervical column injury. Should this patient be decompressed immediately? Or should she be managed conservatively to observe the extent of neurological improvement, with the intention of decompressing her later if necessary? And if she is to have an operation, what operation should that be?
 | When Should the Decompression Be Performed? |
At the outset, it should be recognized that a properly designed and executed prospective randomized or observational study to answer the question of whether early decompression is superior to a delayed decompression in terms of ultimate neurological recovery has not yet been published. The literature on the results of both surgery or conservative treatment for acute central cord syndrome consists of retrospective case series with small patient numbers, inconsistent indications for surgery, and a wide spectrum of reported functional outcomes, and as such, it is difficult to draw strong conclusions from this.
Guest and colleagues attempted to address the question of timing in their retrospective review of 50 patients with central cord injuries, 26 of whom had acute disk herniations or cervical spine fractures/dislocations, and 24 of whom had cervical spondylosis and stenosis without cervical instability, exemplified by the patient presented in this chapter.12 In the latter group of 24 patients with cervical spondylosis and stenosis, those who had an early decompression (within 24 hours of injury) did not have improved motor function as compared with those with a later decompression (more than 24 hours after injury), although those having early surgery experienced a shorter intensive care unit (ICU) and hospital admission. Importantly, an early decompression was not associated with neurological deterioration, thus refuting the claims that immediate intervention was hazardous given the acuteness of the injury to the spinal cord. In those 26 patients with an acute disk herniation or cervical fracture, an early decompression resulted in greater motor recovery, in addition to a shorter ICU and hospital admission. The failure to show a long-term benefit to earlier decompression in the patients with cervical spondylosis echos the results of Chen et al, who also found that, although patients who underwent decompression early enjoyed a faster neurological recovery, at 2 years postinjury their neurological recovery was not significantly different from that of patients treated conservatively.13
Yamazaki and colleagues also attempted to determine if early surgery was beneficial in the setting of acute central cord syndrome, although in their reporting of 47 patients, surgical decompression was only offered to 23 patients who had an “unsatisfactory recovery” in the first few days of injury.14 Of these, 13 patients were decompressed within 2 weeks postinjury, and 10 were decompressed after 2 weeks. Using a logistic regression analysis, the authors reported that earlier surgery promoted a better neurological recovery, although the selection bias of this study clearly limits the strength of this conclusion. Perhaps a more useful observation of this study was that a significantly (and persistently) narrowed anteroposterior diameter of the spinal canal portended a poor neurological prognosis, and therefore the demonstration of this on MRI or CT scanning might be considered to be an indication for an earlier decompression.
 | What Surgical Procedure Should Be Performed? |
Similar to the issue of timing of intervention, the question of which surgical procedure to use in a patient with an acute central cord syndrome again requires consideration of spinal stability. The characteristics of an unstable fracture or ligamentous injury will strongly influence whether an anterior or posterior stabilization procedure (or both) is warranted. In the more common setting of cervical spondylosis and stenosis without spinal instability, the decision making regarding surgical intervention for central cord syndrome is subject to similar arguments that surround the management of cervical spondylotic myelopathy. This complex debate is currently without resolution. Most would agree that ventral compressive pathology from disk herniations/bulges and osteophytes at one or two levels can be addressed appropriately from an anterior approach. In multilevel stenosis, particularly in the setting of a congenitally narrow spinal canal, a posterior decompression may be optimal to avoid having to do a major, multilevel anterior reconstruction. The presence of cervical kyphosis should be appreciated and should serve as a warning against performing a posterior decompression without stabilization. Beyond these basic principles, the optimal surgical management requires a careful, individualized approach for each patient.
 | Authors’ Preferred Method of Surgical Management |
The patient presented earlier in this chapter was initially managed with a rigid cervical collar and full spinal precautions until an unstable spinal injury could be ruled out with appropriate imaging studies. As is the current practice at our institution, the patient was not administered corticosteroids. Because the patient had recovered substantial motor function in the upper extremities and was otherwise normal in the lower extremities, a decision was made to defer surgical decompression and observe the patient for neurological recovery. The patient was stabilized medically, mobilized with the assistance of a physiotherapist, and discharged as an inpatient to a rehabilitation facility. Six weeks later, at the time of her discharge from rehab, her ASIA motor score was 85 (increased from 78) and she had achieved independence in her activities of daily living. The patient was brought electively for a cervical decompression approximately 6 weeks from her initial injury.
For the surgical management, a decision was made to perform a laminoplasty, for several reasons. The patient had a congenitally narrow spinal canal, and the stenosis was felt to be clinically significant at multiple levels, namely C3-4, C4-5, and C5-6. A long decompression posteriorly was therefore felt to be most appropriate. Furthermore, her initial imaging studies had revealed no signs of acute instability, and her upright radiographs revealed that the sagittal spinal alignment was lordotic.
With the head held in a three-pin head clamp, the patient was turned prone; great attention was paid to ensuring that the neck was not extended. Neutral alignment was confirmed with a radiograph. The posterior cervical spine was exposed and an open door laminoplasty was performed. Using a high speed burr, the lamina of C3 to C6 was thinned down to the ligamentum flavum on the left side (open side), whereas on the contralateral side only the outer cortex was removed (hinge side). A small hook was used to release the ligamentum flavum on the left side before elevating the lamina. The interspinous ligament was preserved in continuity between C2-3 and C6-7 to maintain a posterior tension band. Small maxillofacial plates were used to support the “open door.” Local bone was then harvested from the exposed lamina and spinous processes to place on the “hinge side.”
Pearls
Ensure preoperatively that the anesthesiology team is aware of the patient’s spinal cord injury and that the cord will inevitably undergo some manipulation during the decompression, Even in the absence of spinal instability, they should be warned against any hyperextension of the neck during intubation, and it should be emphasized that they be vigilant throughout the entire case about maintaining an adequate mean arterial pressure to keep the cord well perfused.
Because this is a hyperextension injury, great care should be paid while turning the patient prone to keep the neck in a neutral position. A radiograph can confirm this and allow one to change the neck position prior to beginning the exposure. Additionally, a neutral to slightly flexed neck position will make the decompression easier and will facilitate wound closure. If approaching with the intention of not fusing, careful attention should be paid to ensuring that the facet joints and capsules are not violated during the exposure, and that the interspinous ligaments are maintained throughout. Although the exposure requires seeing the top of the C3 lamina, one should attempt to remove as little of the posterior soft tissues as possible from the large spinous process of C2 because this level has a tendency to fall into kyphosis.
When performing the laminoplasty, it is desirable to “open the door” on the most symptomatic side if the patient has presented with asymmetric symptoms or signs. When burring the “troughs” along each side of the lamina, be cautious about starting too laterally because you will enter the lateral masses and be unable to either complete the trough or hinge the lamina open. On the hinge side of the laminoplasty, the tendency with the high-speed burr is to thin the inferior and more dorsal aspect of the lamina while leaving the more superior and ventral aspect. If the hinge side will not crack despite burring the outer cortex, it is likely that the superior aspects of the lamina have been left too thick.
Applying fixation with drills, screws, and screwdrivers around a partially exposed spinal cord is always potentially hazardous. If using maxillofacial reconstruction plates or any of the plates more recently designed specifically for laminoplasty, remember that the fixation is only meant to hold the lamina in an open position until the hinge side heals. It is not an attempt to achieve rigid fixation across the plate.



