Fig. 1
(a) Image of the lid of the phantom with seven 4-cc vials containing serial dilutions of iron concentration in sterile water stuck to the undersurface. (b) MRI of the phantom on a 3 T scanner with following sequence specifications: 3D TR = 40 ms, TE = 6.5, 11, 15.5, 20, 24.5, 29, 33.5, 38 ms, 1.5 mm slice-to-slice, acquired as 3 mm, acquired resolution matrix = 240 × 240, FOV – 240 mm × 240 mm
Table 1
Seven serial dilutions scanned in the MRI phantom with T2* signal measurements corresponding to each concentration
Iron concentration in the vial (mg/ml) | Corresponding MR signal magnitude ± SD |
|---|---|
0.6 | NA |
0.3 | 2.33 ± 0.46 |
0.15 | 3.83 ± 0.14 |
0.075 | 7.99 ± 0.12 |
0.0375 | 15.15 ± 0.29 |
0.01875 | 29.65 ± 0.91 |
0.009375 | 54.77 ± 3.85 |
0.0046875 | 98.23 ± 3.27 |
Human Subjects Scanned
ICH patients, age 18–85 years, with brain parenchymal hemorrhage, no previous ICH, and no evidence of physiological calcification on noncontrast CT of the head were included in the study. One control and two human subjects who met the inclusion criteria for the study were scanned with the same MRI protocol utilized on the phantom. The two human subjects had spontaneous basal ganglia hemorrhage and were scanned on day 7 of their hemorrhage. In the control human brain, MRI regions of interest (ROIs) were drawn in the basal ganglia region on both hemispheres. Calculations were performed on R2* maps generated from the MRI sequences (Fig. 2a). In the two human subjects, regions of interest were drawn on the periphery of the hematoma in the left basal ganglia (Fig. 2b, c). Region of interest measurements were also performed on the contralateral normal hemisphere in an identical anatomical location.
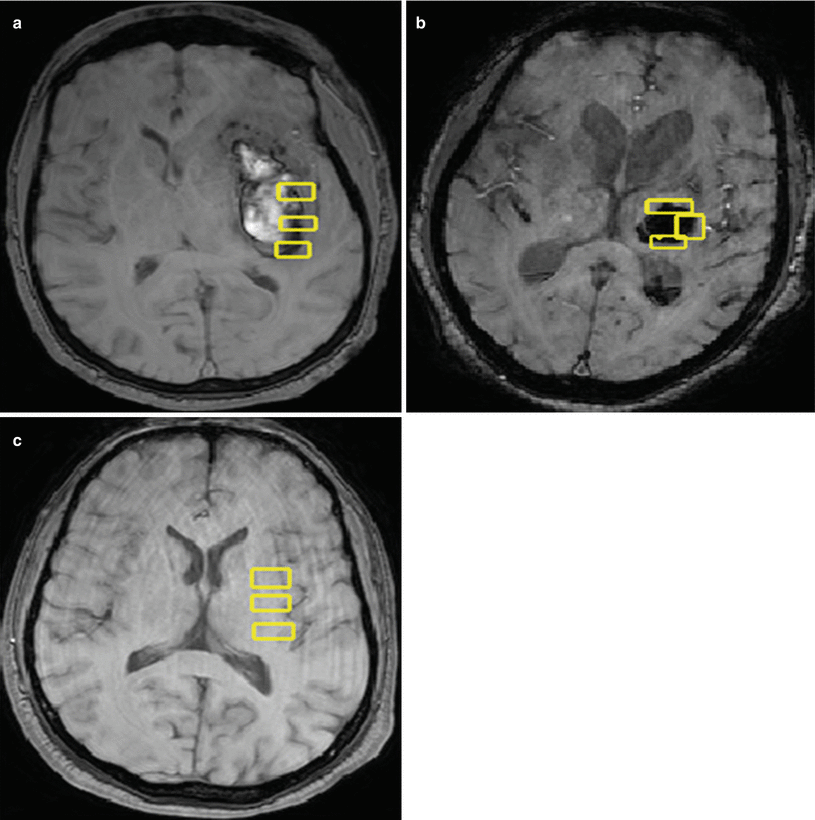

Fig. 2
(a) A 70-year-old male patient with axial MRI brain showing left basal ganglia hemorrhage with regions of interest drawn for measurement of the R2* magnitude. (b) A 68-year-old male patient with axial MRI brain showing left basal ganglia hemorrhage with regions of interest drawn for measurement of the R2* magnitude. (c) A 45-year-old male subject utilized as control showing axial MRI with regions of interest drawn for baseline R2* magnitude measurement
Results
Control Human Subject
In the right basal ganglia, the T2* average of three ROIs measured 44.3. In the left basal ganglia in the same control human subject, the average of three ROIs measured 53.2. Both of these measurements correspond to an iron concentration of 0.01 mg/ml.
First Human Subject with ICH
Perihematomal measurements performed with three ROIs in the left basal ganglia showed T2* values of 15.3. This corresponds to an iron concentration of 0.04 mg/ml. An average of three ROIs in the contralateral normal brain in an identical anatomical location measured 49.5. This corresponds to 0.01 mg/ml of iron concentration.
Second Human Subject with ICH
Perihematomal measurements performed with three ROIs in the left basal ganglia showed T2* values of 18.97. This corresponds to an iron concentration of 0.04 mg/ml. An average of three ROIs in the contralateral normal brain in an identical anatomical location measured 53.3. This corresponds to 0.01 mg/ml of iron concentration.
The above measurements demonstrate consistently that the detection of an iron concentration in the periphery of the hematoma of an ICH in the left basal ganglia on day 7 following the ictus are 4 times higher than the normal baseline concentration.
Conclusion
Our experiment demonstrates proof of principle of MRI being able to detect a 4 times increase in tissue iron levels in the periphery of the hematoma in comparison to baseline. The initial translation from bench to bedside of iron-chelating therapy with deferoxamine being investigated by a phase II trial holds promise. Our hypothesis, once validated in a larger study, can provide a surrogate marker of severity of neurotoxicity following an ICH. Moreover, an MRI-based brain tissue iron quantification may provide a more objective way of monitoring therapy with iron chelates. Furthermore, the correlation of tissue iron quantification with functional outcome following an ICH needs to be studied in a large-scale prospective analysis involving human subjects.
References
1.
2.
Morgenstern LB, Hemphill JC 3rd, Anderson C, Becker K, Broderick JP, Connolly ES Jr et al (2010) Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 41(9):2108–2129, PubMedPubMedCentralCrossRefPubMed
3.
4.
5.
6.
7.









