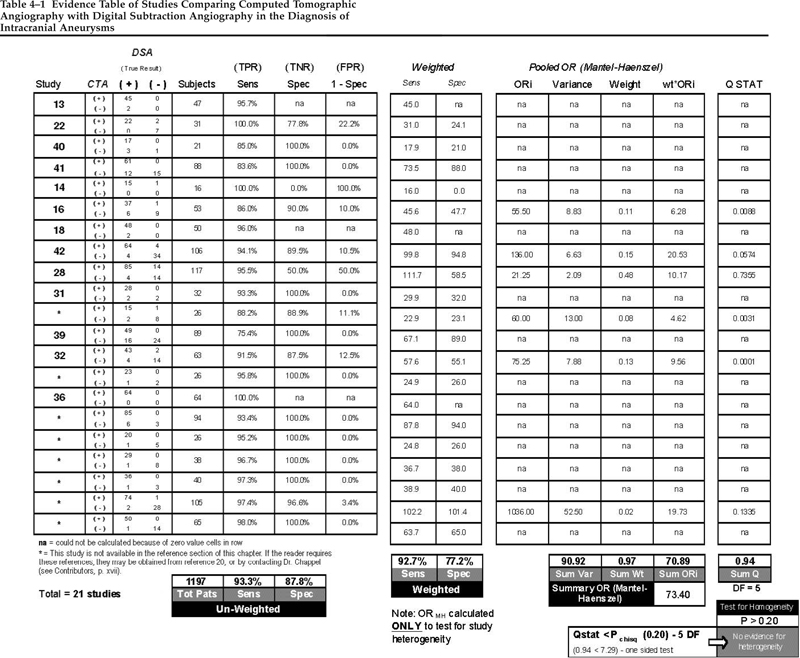
In cerebrovascular surgery, assessment of the patient’s condition can include imaging studies such as obliteration of aneurysm after clipping, increased intravascular size after endarterectomy, absence of intracranial hematoma after evacuation, or reversal of vasospasm on magnetic resonance imaging (MRI). In studies of stroke patients in particular, the clinical examination of patients in terms of their neurological status is essential and this must include all areas covered by the major arteries of the cerebral circulation. Several scales representing the comprehensive neurological examination in stroke have been developed. These include the European Stroke Scale,1,2 the Canadian Neurological Scale,3,4 the Oxbury Initial Severity Scare,5 the Edinburgh Scale,6 and the most frequently used National Institutes of Health (NIH) Stroke Scale, representing a combination of the other scales.1,7–9 The NIH scale was synthesized from the others by Brott and colleagues8 with the objective of covering the areas of the brain served by each of the major arteries. Level of consciousness was described in a range of “alert” to “coma” represented by a numerical score from 0 to 3. Additional aspects of levels of consciousness are further measured by the patient’s response to direct inquiry, graded as “answers both correctly” to “incorrect” and represented by scores of 0 to 2; responses to commands are indicated by sensory examination, limb ataxia, dysarthria, dysphasia, and plantar response. Each category has a varied number of items scored from 0 to 3. A neurologically intact patient exhibits a score of 0. An instruction manual was devised to try to assure acceptable reliability between observers and observations. The original study on the Stroke Scale compared observations by attending neurologists, neurology residents, neurology nurse-clinicians, and emergency department nurse-clinicians. The agreement between observers ranged from substantial (k = ≥0.6) for the Glasgow Coma Scale’s papillary light response and papillary inequality to only moderate agreement (k = 0.4−0.6) for most of the remainder. The sensory exam and extensor plantar response had poor reliability (k = 0.35 and 0.24, respectively).8 Intraobserver and interobserver agreement for the scale as a whole have not been studied. Most existing computed tomographic (CT) scanners, if coupled with the necessary computer hardware and software, can acquire data that can be reformatted to provide angiographic images. This technology, called CT angiography (CTA), has been used in some institutions since the early 1980s. As with any new technology, the user “learning curve,” the resolution of problems, and diligent comparisons with a standard method have slowed the acceptance and widespread use of CTA. Many physicians routinely use CTA in clinical practice, however. In fact, Matsumoto et al10 recently reported on 100 consecutive patients with subarachnoid hemorrhage (SAH) whose aneurysms were successfully treated solely on the basis of CTA. Moreover, many authors have reported multifaceted superiority of CTA over digital subtraction angiography (DSA).11–19 A meta-analysis of the literature comparing CTA with DSA in the diagnosis of intracranial aneurysms has been conducted.20 Twenty reports met the criteria for inclusion in the analysis12,13,20–37; the data extracted from these studies are shown in Table 4–1. The analysis yielded a sensitivity of 0.933 (93.3%; range: 75.4 to 100%) and specificity of 0.878 (87.8%; range: 0 to 100%) for CTA in the diagnosis of cerebral aneurysms. When the studies were weighted for the number of patients in each individual study, the sensitivity dropped slightly to 0.927 (92.7%) and the specificity more substantially to 0.772 (77.2%). Only six of the 20 studies that met the criteria established for the analysis could be used for the statistical methods applied (the Mantel–Haenzel test for homogeneity). However, all 20 studies meet the criteria for class B evidence as defined in the quality of evidence ratings for diagnostic tests developed by the American Academy of Neurology Therapeutics and Technology Subcommittee. Specifically, the data reported in the selected studies were collected prospectively, typically from a relatively small number of patients with the suspected condition (aneurysmal SAH), using the gold standard diagnostic test (DSA) in a blinded fashion. Collectively, studies to date support a strong grade III recommendation when establishing practice guidelines, sometimes referred to as an “option.” This is beneath a grade I recommendation or “standard,” and the similar grade II recommendation, or “guideline.” When rendering classification of evidence of this type, the sensitivity and specificity results also bear on the strength of the recommendation. The studies included in the meta-analysis also involve many early and limited experiences with CTA. Even so, as many as a third of the investigators found sensitivities approaching or equal to 100% and many reported aneurysms detected by CTA that were not seen on DSA.26,32,34,38,39 A recent report describes a per-aneurysm sensitivity of 94.8% and a specificity of 95.2% in 50 patients utilizing multislice CTA. Multislice techniques require a CT scanner with a row of detectors (usually 4, 8, or 16). These higher success rates and increasing comfort with the technology has led some surgeons to treat patients with aneurysmal SAH successfully using CTA alone. On the other hand, there are some disadvantages of CTA. Obtaining software, training personnel, and the need to upgrade CT scanners entail capital outlays and professional initiative. In some cases, CTA requires a larger contrast volume than DSA, particularly if DSA is required after an inconclusive CTA. Many do not consider this a major concern, however, and there are methods to reduce the risk associated with a large dose of contrast.19 With a single intravenous (IV) dose of contrast, CTA provides unlimited viewing angles in three dimensions (3D), whereas DSA typically provides several two-dimensional (2D) views of the vascular pathology. The radiologist and the surgeon can visualize 3D constructs of the cerebral vasculature within minutes at the computer workstation. If the operating room is equipped with a computer on a network connected to the scanner’s workstation, the surgeon can access images directly in the operating room. CTA does not always delineate vessels as small as 1 mm, though one group has reported detecting an 0.8 mm aneurysm.30 However, most vessels of this size are not seen on DSA either. Aneurysms near bony structures may also be partly obscured on CTA, yet this interference can be very useful in understanding the relationship of the aneurysm with the skull base and preoperative planning. In addition, the cerebral veins are often depicted as clearly as the arteries, making interpretation more difficult. Again, information about the veins can also be useful and careful manipulation of the graphics will allow removal of structures that obscure the areas of interest. Finally, information about collateral flow can only be inferred from on CTA; for example, by assessing the patency of the circle of Willis. The disadvantages of using CTA in the diagnosis of cerebral aneurysms are balanced by certain advantages. First is the reduced invasiveness and risk of CTA, which are essentially nil. Whereas some investigators have expressed difficulty detecting aneurysms <3 mm and some <5 mm in diameter,10,29,35,40–42 others have reported the detection of aneurysms <1 mm in diameter by CTA as well as aneurysms that were not seen on DSA.25,31,33,38,39 CTA has been found useful in numerous clinical situations, including cases of SAH highly suspect for aneurysm when DSA is negative.39 Moreover, if a routine head CT scan is performed and found positive for SAH, then follow-up CTA while the patient is still in the scanner is readily accomplished. This may then accelerate admission to an intensive care unit, the operating room, or the interventional radiology suite, possibly reducing the incidence of early recurrent rupture, which sometimes occurs during diagnostic angiography. In a patient with a critical intracerebral hematoma from a ruptured aneurysm, the rapid diagnosis provided by CTA is of major importance. The greatest advantage of CTA, however, lies in the ability to visualize the shape and relationship of aneurysms to adjacent vessels and bony structures in three dimensions and at any angle prior to surgery.13–15,17,18,21,35,36 CTA can also provide useful information about the neck of an aneurysm, including the presence of calcium or atheroma. Information of this nature may even influence preoperative treatment decisions, such as whether to address a lesion by craniotomy or with endovascular techniques.17,18 Although 3D DSA does obviate some of the advantages of CTA, 3D DSA still does not allow visualization of the bony relationships or a clear depiction of calcification or atheromatous disease. As far as other less-invasive diagnostic alternatives go, magnetic resonance angiography (MRA) has been found to be inferior to CTA in virtually every regard.19 Several criteria are applied to natural history studies that seek to determine the prognosis of a health condition or disease. These include a well-defined and representative sample of patients assembled at a common and early point in the course of their disease, sufficiently long and complete patient follow-up, objective outcome criteria, and adjustment for any previously validated prognostic risk factors. When all criteria are met for a prospective study of unselected patients (including nontreatment or control arms of randomized trials) then the findings of the study, or “evidence” is considered class I. Class II evidence is based on either selected patients identified and followed prospectively in an observational cohort or similar and highly reliable retrospective studies (such as well designed case-control studies); class III evidence is more anecdotal, coming from retrospectively examined case-series or expert opinions. The natural history of unruptured intracranial aneurysms has been the subject of several reviews.43–46 These reviews, combined with a MEDLINE search conducted for 1990 to 2003 (English articles), yielded 10 original articles on the prognosis of unruptured intracranial saccular aneurysms suitable for the present analysis (Table 4–2). Most studies have found an annual rupture risk or rate between 1 and 2%, although additional risk factors must be considered that influence the rate, including aneurysm size, history of SAH from another treated aneurysm, aneurysm location, hypertension, and cigarette smoking. In the case of unruptured intracranial aneurysms, treatment recommendations are based not just on prognosis for rupture and the consequences of rupture but also on the anticipated risks of the different treatments available. Treatment standards, which are management recommendations indicating a high degree of certainty that the treatments are valid, must be supported by class I natural history data, and this type of information does not yet exist for unruptured intracranial aneurysms. Instead, only management guidelines and options can be formulated based on the class II and III evidence to be discussed, along with an understanding of the risks involved in treatments provided. Such recommendations are available in the reviews mentioned above. Table 4–2 summarizes 10 studies on the natural history of unruptured intracranial aneurysms published between 1981 and 2003.47–56 All are observational studies of patients possessing unruptured aneurysms that were not repaired. The largest of these studies, and deserving of some description and comment, was the International Study of Unruptured Intracranial Aneurysms (ISUIA), a multicenter study designed specifically to help define the natural history of unruptured intracranial aneurysms as well as risk associated with their repair. The first part of ISUIA assessed the natural history of 1937 unruptured aneurysms in 1449 patients from 53 centers in the United States, Canada, and Europe.53 It was a retrospective examination of patients whose aneurysm was diagnosed between 1970 and 1991 and for whom medical records and hard copies of angiograms were available. Of the 1449 patients, 727 patients had no history of SAH (designated “group 1”) and 722 had a history of SAH from a different aneurysm that had been successfully repaired (group 2). The rupture rate was calculated to be ~0.05% per year for patients with small aneurysms <10 mm in size in group 1 (with no history of SAH), and the rate was between 0.5 and 1% for the remaining patients (group 1 patients with aneurysms >9 mm and group 2 patients with prior SAH). Aneurysm size and location were independent predictors of rupture, and giant aneurysms (≥25 mm in diameter) as well as those located in the posterior circulation or on the posterior communicating artery were at highest risk. There was an unexplained low incidence of anterior cerebral artery aneurysms in this retrospective analysis (10%), and nearly 17% of the aneurysms included were located in the cavernous sinus. When the latter group of cavernous aneurysms, well recognized to have a relatively benign natural history, was excluded from the analysis, the overall rupture risk results did not change. The low rupture risks in this historical cohort of ISUIA, especially in the smaller aneurysms found in patients without a prior SAH history, caused a sensation in the neurosurgical community. However, these results were found in patients selected by their treating physicians for no aneurysm repair, thereby possibly excluding patients with a higher risk of rupture and introducing data collection bias into the results (the evidence class II).  4
4 
Cerebrovascular Surgery
J. Max Findlay, E. Thomas Chappell
Patient Assessment
Establishing the Diagnosis
Computed Tomographic Angiography for the Diagnosis of Cerebral Aneurysms
Determination of Prognosis
Natural History Studies
UNRUPTURED INTRACRANIAL ANEURYSMS
Literature Review
RUPTURE RISK
| Study and Year | Class of Evidence | Number of Unruptured Aneurysms | Mean Follow-Up | Annual Rupture Rate (%) | Risk Factors for Ruptur |
|---|---|---|---|---|---|
| Locksley, 199647 | II | 34 | 47 months | 7 | Size >7 mm |
| Wiebers et al, 198148 | II | 81 | 98.5 months | See Weibers49 | Size >9 mm |
| Wiebers et al, 198749 | II | 161 | 8.3 years (1079 patient years) | 0 for <10 mm, 5.9% for > 9 mm | Size >9 mm |
| Asari and Ohmoto, 199350 | II | 72 | 43.7 months | 1.9% | HPT, site, and shape |
| Taylor et al, 199551 | II | 20,767 | 30 months | Between 1 and 2% | HPT |
| Yasui et al, 199752 | III | 303 | 75.1 months | 2.3% | None |
| ISUIA, 1998 (Retrospective Cohort)53 | II | 1937 | 8.3 years | <10 mm, no prior SAH: 0.05%; others ~1% | Prior SAH, size >9 mm, Pcomm and VB location |
| Juvela et al, 200054 | 2 | 181 | 19.7 years (2575 patient years) | 1.3% | Size >7 mm, younger age (continuous variable), cigarette smoking |
| Tsutsumi et al, 200055 | 2 | 62 | 4.3 years | 2–3% | Size |
| ISUIA, 2003 (Prospective Cohort)56 | 2 | 2686 | 4.1 years (6544 patient years) | <7 mm, no prior SAH: 0.1%, others ~1%, higher the first year (~7%) and in the presence of risk factors | Prior SAH for aneurysms <7 mm, size >7 mm, basilar tip and Pcomm site |
Abbreviations: HPT, hypertension; Pcomm, posterior communicating; SAH, subarachnoid hemorrhage; VB, vertebrobasilar.
The results of the second part of ISUIA included a prospective follow-up on 1692 patients possessing 2686 unruptured aneurysms diagnosed between 1991 and 1998 that were not repaired.56 In this prospective cohort at a mean follow-up of 4.1 years it was calculated that the annual rupture rate for aneurysms less than 7 mm in patients with no prior history of SAH was 0.1%. This rupture rate was significantly higher and the threshold aneurysm size significantly smaller than suggested by the previous retrospective results of ISUIA. For aneurysms ≥7 mm in size a prior history of SAH did not affect prognosis, although increasing aneurysm size and location in the posterior circulation did. For aneurysms ≥7 mm in diameter, with or without prior SAH, the risk increased from 0.5 to ~1% per year, although the risk was considerably higher in the first year following entry into the study for aneurysms ≥10 mm in diameter (7% for aneurysms 10–24 mm, 17% for aneurysms >24 mm). Although this second part of ISUIA was prospective, the patients were again deliberately selected, for unspecified reasons and at the discretion of the treating physicians, to not receive treatment for their aneurysms. The results of the prospective arm of ISUIA are therefore again subject to bias, and the conclusions of this study are class II evidence. Even so, the results of the second part of ISUIA are more consistent with the rest of the natural history literature.
RISK FACTORS FOR RUPTURE
Additional risk factors for aneurysm rupture must be considered for individual patients, including increasing aneurysm size (associated with an increased rupture risk), associated nonrupture symptoms (i.e., cranial nerve compression; increased risk), location either on the posterior communicating artery or at the basilar tip (increased risk), cigarette smoking (increased risk), arterial hypertension (increased risk of rupture and possibly death from rupture), a history of SAH from another treated aneurysm (increased risk), and a longer or multilobed aneurysm shape (increased risk).43,57,58 Whether or not a family history of aneurysms increased the risk of rupture is not clear.
CONSEQUENCES OF RUPTURE
When considering the prognosis of unruptured intracranial aneurysms, not just the risk of rupture but also the consequences of bleeding must be considered. In ISUIA the mortality rate associated with aneurysm rupture was ~66%, a figure in keeping with what is generally known about the mortality associated with aneurysm rupture.53,56 Although the annual risk of rupture from an intact aneurysm is usually very low, the consequences of each rupture are high.
CUMULATIVE RISK OF RUPTURE
As discussed, the annual rupture risk of an unruptured intracranial aneurysm greater than 7 mm in diameter is ~1%. The most relevant topic in a clinical discussion with patients is the risk of bleeding, either a cumulative or a lifetime risk—the estimation of which requires life-table actuarial methods. Specifically, the equation of cumulative probability is used to calculate risk of bleeding over a finite time, including estimated years to live. The equation is 1 – ny = P, where n is the yearly chance of not having the event (in the case of unruptured aneurysm rupture, between 0.97 and 0.99), y is the number of years over which the event is to be considered (which may be the years estimated of remaining life), and P is the cumulative risk of the event over the period of time y. For example, assuming an annual risk of rupture of 1% from an incidental aneurysm in an otherwise healthy young male aged 45, the lifetime risk of bleeding is 28.2%, assuming a life expectancy to age 78. If that same patient possesses additional risk factors for aneurysm rupture, such as an aneurysm location at the basilar tip, a larger aneurysm size, and cigarette smoking, for example, and the annual risk is considered higher at 3%, then the lifetime risk increases to 63.4%. For many patients, however, with significant comorbidities or risk factors such as smoking, it is more realistic to think in terms of either a 5- or 10-year risk of hemorrhage because related conditions such as heart or lung disease may have a significant bearing on life expectancy.
MANAGEMENT DECISIONS FOR UNRUPTURED INTRACRANIAL ANEURYSMS
Finally, the risk of aneurysm rupture and spontaneous mortality and morbidity must be balanced against the risk of intervention for aneurysm repair when considering treatment. As shown in ISUIA, the risks of treatment, both surgical and endovascular, are considerable, with rates of moderate-to-severe neurological disabilities (Rankin scores of 3, 4, or 5) between 10 and 15%.53,56 Risk factors for poor outcome from treatment included age >50 years, size >25 mm, location in the posterior circulation, nonrupture symptoms arising from the aneurysm (compression or ischemia), and the presence of ischemic cerebrovascular disease (i.e., prior stroke). Recommendations for treatment for patients with unruptured intracranial aneurysms must be made on a patient-to-patient basis using all of the information available.
Evaluation of Intervention
Prevention
CAROTID ENDARTERECTOMY IN STROKE PREVENTION
The performance of carotid endarterectomy (CEA) reached peak rates in the mid-1980s, but although enthusiasm for the procedure was high at that time, evidence of its efficacy was low. Rates fell rapidly as doubts about the benefits and appropriateness of CEA grew.59–61 Following the release of randomized clinical trial results positive for CEA, surgery rates in Canada and the United States recovered quickly in the 1990s,62–67 recorded to be between 100 to 400 per 100,000 health care beneficiaries in certain U.S. states,64,66 and 26 to 83 per 100,000 population among Canada’s provinces.67 This increased performance on CEA has renewed interest in the most effective investigation of carotid stenosis, the selection and risk stratification of patients for surgery, treatment outcomes, and the various anesthetic and technical aspects of the procedure. Surgical efficacy, optimal patient selection for surgery, and the effectiveness of CEA will be the focus of this section.
EFFICACY OF CAROTID ENDARTERECTOMY FOR SYMPTOMATIC CAROTID STENOSIS
In the past 15 years three randomized trials have been completed evaluating the benefit of CEA for patients with symptomatic stenosis of the internal carotid artery68–72; their main results are summarized in Table 4–3. These results have also been pooled for a collective reanalysis.73 The European Carotid Surgery Trial (ECST) used a different method of stenosis calculation, so this study must be interpreted accordingly.70,71 The method of calculating carotid stenosis in the North American trials, and the method used in the pooled analysis73 compares the narrowest diameter of the residual lumen (on the angiographic view showing the greatest stenosis) (N) to the luminal diameter of the internal carotid artery well beyond the bulb where the walls of the artery have become parallel (D), the percentage stenosis calculated as (1− N/D) ×100.74 The exclusion criteria for the two large trials, the North American Symptomatic Carotid Endarterectomy Trial (NASCET) and ECST were similar; patients were excluded if they (1) had an intracranial lesion that was more severe than the surgically accessible lesion; (2) had a cerebral infarction that deprived them of all useful function in the affected territory; or (3) were 80 years of age or older. Patients were temporarily ineligible if they: (1) had uncontrolled hypertension, diabetes mellitus, or unstable angina; (2) had had a myocardial infarction within the previous 6 months; or (3) had signs of progressive neurological dysfunction. The Veterans Affairs Cooperative Study of Symptomatic Stenosis72 had randomized only 189 patients when it was terminated following the positive early results from NASCET and ECST. At a mean 1-year follow-up, surgery resulted in a reduction of the combined end points stroke and transient ischemic attack (TIA; p = 0.011), but not stroke alone, and the 30-day stroke or death rate in the surgical group was 6.5%.
| Trial | Institution(s) | No. of Patients | Main Trial Result(s) | 30-Day Surgical Ipsilateral Stroke or Death Rate |
|---|---|---|---|---|
| NASCET68 70–99% Stenosis | 50 centers in the US and Canada | 331 medical 328 surgical | Ipsilateral stroke risk 26% medical, 9% surgical over 2 years, ARR 17% (p < 0.001) | 5.8% |
| ECST70 70–99% & 0–29% Stenosis* | 80 centers in 14 European countries | 70–99%: 323 medical 455 surgical 0–29% 155 medical 219 surgical | 70–99%: stroke or death 22% medical, 12% surgical over 3 years, ARR 10% (p < 0.01) 0–29%: surgery harmful | 70–99%: 7.5% 0–29%: 4.6% |
| VA Coop. Study72 50–99% stenosis (men only) | 16 VA medical centers (US) | 98 medical 91 surgical | Stroke or crescendo TIAs 19% medical, 8% surgical over 1 year, ARR 12% (p = 0.01) | 7.7% |
| ECST71 30–69% stenosis* | 97 centers in 15 European countries | 631 medical 959 surgical | Ipsilateral stroke or death over 4 years surgery harmful | 8% |
| NASCET69 50–60% stenosis & <50% stenosis | 50 centers in the US and Canada | 50–69%: 428 medical 430 surgical 0–49%: 690 medical 678 surgical | 50–69%: Ipsilateral stroke 22% medical, 6% surgical over 5 years, ARR 6.5% (p = 0.045) 0–49%: No significant difference | 6.7% |
*30% ECST stenosis is roughly equivalent to 16% NASCET stenosis, and 70% ECST stenosis is roughly equivalent to 50% NASCET stenosis.48
Abbreviations: NASCET, North American Symptomatic Carotid Endarterectomy Trial; ECST, European Carotid Surgery Trial; VA Coop Study, Veterans Cooperative Study; TIA, transient ischemic attack; ARR, absolute risk reduction.
The 5-year results from NASCET are available75 and are summarized in Table 4–4. In assessing the results of the randomized trials for CEA emphasis has been placed on the “number needed to treat” (NNT) statistic, which is the number of CEAs that need to be performed to prevent a single stroke over a designated time period for a certain patient group. Calculated as the inverse of the absolute risk reduction (ARR) provided by the intervention (in this case endarterectomy), it is a practical way to judge the relative efficacy of CEA in different patient groups, such as those with different degrees of stenosis. Since the publication of these studies, sufficient time has passed to allow for their thorough analysis; several organizations and experts have published their own guidelines for the use of CEA.75–85
Carotid endarterectomy is highly appropriate for patients with symptomatic, severe (70 to 99%) stenosis causing either TIAs or nondisabling stroke, a direct determination of all three randomized controlled trials and so a grade IA or grade I recommendation. The maximum allowable rate of all strokes or death in this group of patients is 6%. Patients with symptomatic stenosis in the 50 to 69% range benefit less from surgery and are uncertain candidates for CEA in general. Some of these patients, however, will benefit if selected based on additional features that indicate a higher stroke risk when the stenosis is treated with medical therapy alone. Determined from subgroup analyses within the randomized trials (and therefore not directly addressed in the design of the trial) the factors that when present enhance the benefit of CEA include: (1) male sex,69 (2) a hemisphere as opposed to retinal presentation,86 (3) a stroke as opposed to TIA presentation,69 (4) a higher degree of stenosis,69,73,87 (5) plaque ulceration,87–89 (6) contralateral carotid occlusion,90 (7) the presence of intraluminal thrombus,91 (8) the presence of intracranial (“tandem”) atherosclerosis,92 (9) the absence of collateral pathways to the distal internal carotid artery,93 and (10) the presence of leukoaraiosis (white matter changes) on brain CT scanning.94 A practical approach to patients with a moderate and symptomatic carotid stenosis is to evaluate them for the presence of the aforementioned features and, to consider surgery only if at least one feature is present. Surgery is more strongly indicated in patients with the greatest number of additional risk factors (grade II recommendation).
Patients who are inappropriate candidates for CEA are those with less than 50% stenosis and those with unstable medical or neurological conditions such as unstable angina, recent myocardial infarction, uncontrolled congestive heart failure, or a progressing or major stroke.
EFFICACY OF CAROTID ENDARTERECTOMY FOR ASYMPTOMATIC STENOSIS
Two randomized, controlled trials have been completed that address CEA in asymptomatic patients, and they are summarized in Table 4–5. The methodology of another trial precluded any conclusions being drawn about the efficacy of CEA,95 and another trial for asymptomatic stenosis was terminated because of excessive cardiac morbidity in the surgical arm.96
| Risk of Ipsilateral Stroke | |||||||
|---|---|---|---|---|---|---|---|
| Stenosis%* | No. of Patients | Medical | Surgical | Absolute Risk Reduction | Relative Risk Reduction% | NNT | 30-Day Perioperative Stroke and Death Rate% |
| 70–99 | |||||||
| 2 years | 659 | 24.5 | 8.6 | 15.9 | 65 | 6 | 5.8 |
| 5 years | 575 | 28.0 | 13.0 | 16.4 | 54 | 6 | 5.8 |
| 50–69 | |||||||
| 2 years | 858 | 14.6 | 9.3 | 5.3 | 36 | 19 | 6.9 |
| 5 years | 858 | 22.2 | 15.7 | 6.5 | 29 | 15 | 6.9 |
| <50 | |||||||
| 2 years | 1368 | 11.7 | 10.2 | 1.5 (NS) | 13 | 67 | 6.5 |
| 5 years | 1368 | 18.7 | 14.9 | 3.8 (NS) | 20 | 26 | 6.5 |
*Stenosis according to NASCET measurement method.74
Abbreviations: NASCET, North American Symptomatic Carotid Endarterectomy Trial68,69,75; NNT, the number (of patients) needed to treat (by endarterectomy to prevent one additional ipsilateral stroke in either 2 or 5 years, as shown, after the procedure compared with medical therapy alone); NS, not significant.
| Risk of Ipsilateral Stroke | |||||||
|---|---|---|---|---|---|---|---|
| Study | No. of Patients | Medical | Surgical | Absolute Risk Reduction | Relative Risk Reduction% | NNT | 30-Day Perioperative Stroke and Death Rate% |
| VA Study97 (men only, >50% stenosis, 4 year follow-up) | 444 | 9.4 | 4.7 | 4.7 (NS) | 50 | 21 | 4.6 |
| ACAS98 (≥60% stenosis, estimated 5 year follow-up) | 1662 | 11.0 | 5.1 | 5.9 | 53 | 17 | 1.2 |
Abbreviations: NNT, the number (of patients) needed to treat (by endarterectomy to prevent one ipsilateral stroke in the number of years after the procedure shown compared with medical therapy alone); VA Study, Veterans Affairs Cooperative Study; ACAS, Asymptomatic Carotid Atherosclerosis Study; NS, not significant.
The efficacy of CEA for treatment of asymptomatic stenosis was tested in a randomized trial that involved 444 men in 11 U.S. Veterans Administration Hospitals.97 At ~4 years follow-up the surgical group experienced fewer neurological events than controls (8% in the surgical group and 20.6% in the control group, p < 0.001), but these events included translent ischemic attacks (TIAs). When just stroke and death combined were examined, there was no significant difference between groups.
The results of a much larger trial, the Asymptomatic Carotid Atherosclerosis Study (ACAS), were published in 1995.98 The 1662 enrolled patients had a stenosis of at least 60% as indicated by carotid Doppler or angiography. At a median follow-up of 2.7 years, Kaplan-Meier estimates of 5-year risks were calculated, and the results showed a benefit for the group that received surgery. The 5-year rate of ipsilateral stroke and death, combined with any postoperative stroke and death for those who had undergone CEA, was calculated to be 5.1% for the treated patients and 11% for the controls (p = 0.004). Although the reduction was statistically significant, the absolute risk reduction was relatively small (6%). In addition, the risk of major stroke or death did not differ significantly between groups, and women did not appear to benefit from surgery. The reason for this gender difference is uncertain, but it may be partly explained by a higher perioperative complication rate among women (3.6%) than men (1.7%). To prevent a single stroke from an asymptomatic stenosis over 2 years, 67 patients need to undergo CEA. The NNT for a 5-year period falls to 17, but to accomplish this objective the risk of perioperative stroke and death needs to be in the range of 1.5%, the very low rate achieved in ACAS after exclusion of all angiography-related strokes.
Asymptomatic patients benefit substantially less from CEA, and for any benefit at all surgery must be performed with particularly low stroke rates, in the range of 2 or 3% (at least one half the stroke rate for symptomatic patients). Evidence from nonrandomized, observational studies has suggested additional risk factors that may increase the risk of an ipsilateral stroke from asymptomatic carotid stenosis (levels III and IV evidence). These include male sex,98 a higher degree of stenosis,99,100 ipsilateral brain infarction on CT or MRI,101 plaque ulceration,102–106 the presence of an occluded contralateral carotid artery,107 a stenosis that worsens over time,108 a partly echolucent or heterogeneous (“soft”) plaque or evidence of intraplaque hemorrhage on ultrasound,108–112 and the presence of microemboli detected on transcranial Doppler ultrasound.113
Asymptomatic stenosis is an uncertain indication for CEA; hence, surgery should only be considered a management option by expert surgeons with very low complication rates in the presence of one or more of the aforementioned risk factors (grade II recommendations).
Asymptomatic patients who are inappropriate candidates for CEA are those with less than 60% stenosis and those with unstable medical or neurological conditions, such as unstable angina, recent myocardial infarction, uncontrolled congestive heart failure, or progressing major stroke.
Guidelines for the performance of CEA are given in Table 4–6.
EFFECTIVENESS OF CAROTID ENDARTERECTOMY
The effectiveness of a medical intervention is the equivalent of efficacy, but measured in the “real world” of clinical practice as opposed to a clinical trial.114 Whereas efficacy studies (i.e., randomized controlled trials) can tell us if a procedure can work, effectiveness studies tell us if it does work in routine clinical practice.
To achieve maximum effectiveness from CEA, which is the greatest number of strokes prevented in the clinical application of this procedure, two conditions must be met. The first is a reduction of perioperative morbidity and deaths to a minimum. This maximizes the ARR for stroke, leaving it limited only by the risk of stroke if the stenosis was treated medically. This then leads to the second condition, which is maximizing the appropriateness of patient selection for CEA by choosing those patients at greatest risk of stroke and with the most to gain by CEA. These patients have the highest baseline risk of stroke treated medically, the largest ARR with surgery, and the smallest NNT to prevent a single stroke over a given number of years. The most effective use of CEA would therefore be in patients at highest risk of stroke without surgery when the operation is performed with the lowest complication rates.
Although methodological shortcomings are unavoidable in retrospective reviews, several recent regional and statewide surveys have reported acceptable CEA stroke or death complication rates for symptomatic patients, ranging between 3 and 7.5%.66,115–118 Patient selection for CEA has been more variable, as have the criteria for appropriate indications. Particularly variable is designation of asymptomatic patients as “uncertain” or “appropriate.”119–123 There has also been marked geographical variation in the rate of CEA between countries and regions within countries, most likely reflecting regional clinical practice and supply of services.64,66,67
| Appropriate Patients |
| Symptomatic 70–99% stenosis |
| Uncertain Patients (Careful Patient Selection Required) |
| Symptomatic 50–69% stenosis |
| Asymptomatic 60–99% stenosis |
| Inappropriate Patients |
| <50% symptomatic stenosis |
| <60% asymptomatic stenosis |
| Unstable medical or neurological status |
| Recent large cerebral infarction |
| Decreased level of consciousness |
| Surgically inaccessible stenosis |
CEA is well suited for examination of its outcomes, complications, and appropriateness. Indications for CEA are well defined, and the major events that can complicate CEA are readily detected when hospital records are reviewed and patients are contacted for follow-up. A regular CEA auditing process implemented in a major Canadian city providing direct feedback of surgical indications and operative results to operating surgeons has been found to result in significant improvements in the effectiveness of CEA, with both surgical indications and results improving over time.123
Conclusion
The investigation, diagnosis, prognosis and treatment of cerebrovascular diseases have been fertile ground for research, yielding abundant evidence on which we currently base sound management recommendations. In this chapter, we have demonstrated that CT angiography is a superb new method of diagnosing intracranial aneurysms, that the prognosis of unruptured intracranial aneurysms is complex but sufficiently quantifiable to be useful in a bedside conversation with patients, and that the indications for surgical repair of carotid stenosis have never been so clear. Present studies are exploring a myriad of imaging techniques, are defining the natural history of other common cerebrovascular disorders such as brain vascular malformations, and are comparing surgical versus endovascular treatments of conditions such as carotid stenosis and intracranial aneurysms. Sir William Osler (1849–1919 said, “It is astonishing with how little reading a doctor can practice medicine, but is not astonishing how badly he may do it.” In our current medical era, good evidence-based reviews are both the least and most a physician should read.
Acknowledgments
The authors would like to thank Ms. Terri Lord for assistance in the preparation of this chapter.
References
- Goldstein LB, Bertels C, Davis JN. Interrater Reliability of the NIH Stroke Scale. Arch Neurol 1989;46:660–662
- Hantson L, De Weerdt W, De Keyser J, et al. The European Stroke Scale. Stroke 1994;25:2215–2219
- Cote R, Battista RN, Wolfson C, Boucher J, Adam J, Hachinski VC. The Canadian Neurological Scale: validation and reliability assessment. Neurology 1989;39:638–643
- Cote R, Hachinski VC, Shurvell BL, Norris JW, Wolfson C. The Canadian Neurological Scale: a preliminary study in acute stroke. Stroke 1986;17:731–737
- Oxbury JM, Greenhall RC, Grainger KM. Predicting the outcome of stroke: acute cerebral infarction. Br Med J 1975;3:125–127
- Sugiura K, Muraoka K, Chishiki T, Baba M. The Edinburgh-2 Coma Scale: a new scale for assessing impaired consciousness. Neurosurgery 1983;12:411–415
- Albanese MA, Clarke WR, Adams HP, Woolson RF, Investigators TOAST. Ensuring reliability of outcome measures in multicenter clinical trials of treatments for acute ischemic stroke. Stroke 1994;25:1746–1751
- Brott T, Adams HP, Olinger CP, et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke 1989;20:864–870
- Lyden P. Brott, Tilley B, Welch KMA, Mascha EJ, Levine S, Haley EC, Grotta J, Marler JR. The NINDS TPA Stroke Study Group: improved reliability of the NIH Stroke Scale using video training. Stroke 1994;25:2220–2226
- Matsumoto M, Sato M, Nakano M, et al. Three-dimensional computerized tomography angiography-guided surgery of acutely ruptured cerebral aneurysms. J Neurosurg 2001;94:718–727
- Kato Y, Sano H, Katada K, et al. Application of three-dimensional CT angiography (3D-CTA) to cerebral aneurysms. Surg Neurol 1999;52:113–122
- Adams HP Jr, Adams RJ, Brott T, et al. Guidelines for the early management of patients with ischemic stroke. Stroke 2003;34:1056–1083
- Alberico RA, Patel M, Casey S, Jacobs B, Maguire W, Decker R. Evaluation of the circle of Willis with three-dimensional CT angiography in patients with suspected intracranial aneurysms [see comments] AJNR Am J Neuroradiol 1995;16:1571–1578
- Dillo W, Brassel F, Becker H. Possibilities and limitations of CT angiography in comparison to DSA in intracranial aneurysm. Rofo Fortschr Geb Rontgenstr Neuen Bildgeb Verfahr 1996;165:227–231
- Dorsch NW, Young N, Kingston RJ, Compton JS. Early experience with spiral CT in the diagnosis of intracranial aneurysms. Neurosurgery 1995;36:230–238
- Eberhardt KE, Tomandl B, Nomayr A, Huk WJ. Value of CT-angiography in the diagnosis of cerebral artery aneurysms. Radiologe 1997;37:905–912
- Hochmuth A, Spetzger U, Schumacher M. Comparison of three-dimensional rotational angiography with digital subtraction angiography in the assessment of ruptured cerebral aneurysms. AJNR Am J Neuroradiol 2002;23:1199–1205
- Hope JK, Wilson JL, Thomson FJ. Three-dimensional CT angiography in the detection and characterization of intracranial berry aneurysms [see comments]. AJNR Am J Neuroradiol 1996;17:439–445
- Ng SH, Wong HF, Ko SF, et al. CT angiography of intracranial aneurysms: advantages and pitfalls. Eur J Radiol 1997;25:14–19
- Chappell ET, Moure FC, Good MC. Comparison of computed tomographic angiography with digital subtraction angiography in the diagnosis of cerebral aneurysms: a meta-analysis. Neurosurgery 2003;52:624–631
- Abe T, Hirohata M, Tanaka N, et al. Clinical benefits of rotational 3D angiography in endovascular treatment of ruptured cerebral aneurysm. AJNR Am J Neuroradiol 2002;23:686–688
- Anderson, GB, Findlay, JM, Steinke, DE, Ashforth, R. Experience with computed tomographic angiography for the detection of intracranial aneurysms in the setting of acute subarachnoid hemorrhage. Neurosurgery 1997;41:522–528
- De Araujo IS. Three-dimensional computed tomographic angiography as preoperative examination in the treatment of cerebral aneurysms [in Portuguese]. Arq Neuropsiquiatr 1998;56:798–802.
- Grossi G, Romanzi G, Macchia G, Ruffinengo U, Calia S. A proposal for emergency diagnosis in subarachnoid hemorrhage as a preliminary to therapeutic choices. Intervent Neuroradiol 1995;1:43–57
- Hashimoto H, Iida J, Hironaka Y, Okada M, Sakaki T. Use of spiral computerized tomography angiography in patients with subarachnoid hemorrhage in whom subtraction angiography did not reveal cerebral aneurysms. J Neurosurg 2000;92:278–283
- Heffez DS, Mikhael M, Jensen K. Operative confirmation of three-dimensional computed tomographic and magnetic resonance imaging of cerebrovascular pathology. J Image Guid Surg 1995;1:179–190
- Ikeda K, Iwasaki Y, Murakami S, Ichikawa Y. Brain 3 D-CT angiography was a useful tool for diagnosis of internal carotid-posterior communicating artery aneurysm: a case of false negative 3 D-MRA. No To Shinkei 1999;51:805–808
- Imakita S, Onishi Y, Hashimoto T, et al. Subtraction CT angiography with controlledorbit helical scanning for detection of intracranial aneurysms. AJNR Am J Neuroradiol 1998;19:291–295
- Iwanaga S, Shrier DA, Okawara SH, Numaguchi Y. Value of CT angiography in the evaluation of a peripheral anterior inferior cerebellar artery aneurysm: case report. Clin Imaging 1999;23:77–80
- Jansen O, Braks E, Hahnel S, Schramm T, Sartor K. CT angiography to determine the size of intracranial aneurysms before GDC therapy. Rofo Fortschr Geb Rontgenstr Neuen Bildgeb Verfahr 1998;169:175–181
- Koch C, Grzyska U, Probst EN, et al. CT-angiography for diagnostic assessment of intracranial vascular aneurysms. Wien Med Wochenschr 1997;147:163–171
- Lenhart M, Bretschneider T, Gmeinwieser J, Ullrich OW, Schlaier J, Feuerbach S. Cerebral CT angiography in the diagnosis of acute subarachnoid hemorrhage. Acta Radiol 1997;38:791–796
- Louis BM, Hoch BS, Hernandez C, et al. Protection from the nephrotoxicity of contrast dye. Ren Fail 1996;18:639–646
- Marro B, Zouaoui A, Koskas F, et al. Computerized tomographic angiography scan following carotid endarterectomy. Ann Vasc Surg 1998;12:451–456
- Ng SH, Wong HF, Ko, SF et al. CT angiography of intracranial aneurysms: advantages and pitfalls. Eur J Radiol 1997;25:14–19
- Ogawa T, Okudera T, Noguchi K, et al. Cerebral aneurysms: evaluation with three-dimensional CT angiography [see comments]. AJNR Am J Neuroradiol 1996;17:447–454
- Ohkawa M, Tanabe M, Toyama Y, et al. CT angiography with helical CT in the assessment of acute stage of subarachnoid hemorrhage. Radiat Med 1998;16:91–97
- Kiyosue H, Okahara M, Tanoue S, Nakamura T, Nagatomi H, Mori H. Detection of the residual lumen of intracranial aneurysms immediately after coil embolization by three-dimensional digital subtraction angiographic virtual endoscopic imaging. Neurosurgery 2002;50:476–485
- Lai PH, Yang CF, Pan HB, Chen C, Ho JT, Hsu SS. Detection and assessment of circle of Willis aneurysms in acute subarachnoid hemorrhage with three-dimensional computed tomographic angiography: correlation with digital subtraction angiography findings. J Formos Med Assoc 1999;98:672–677
- Anderson GB, Steinke DE, Petruk KC, Ashforth R, Findlay JM. Computed tomographic angiography versus digital subtraction angiography for the diagnosis and early treatment of ruptured intracranial aneurysms. Neurosurgery 1999;45:1315–1323
- Aoki S, Sasaki Y, Machida T, Ohkubo T, Minami M. Cerebral aneurysms: detection and delineation using 3-D-CT angiography. AJNR Am J Neuroradiol 1992;13:1115–1120
- Hsiang JN, Liang EY, Lam JM, Zhu XL, Poon WS. The role of computed tomographic angiography in the diagnosis of intracranial aneurysms and emergent aneurysm clipping. Neurosurgery 1996;38:481–486
- Rinkel GJE, Djibuti M, Algra A, van Gijn J. Prevalence and risk of rupture of intracranial aneurysms: a systematic review. Stroke 1998;29:251–256
- Bederson JB, Awad IA, Wiebers DO, et al. Recommendations for the management of patients with unruptured intracranial aneurysms. Stroke 2000;31:2742–2750
- Brennan JW, Schwartz ML. Unruptured intracranial aneurysms: appraisal of the literature and suggested recommendations for surgery, using evidence-based medicine criteria. Neurosurgery 2000;47:1359–1372
- Weir B. Unruptured intracranial aneurysms: a review. J Neurosurg 2002;96:3–42
- Locksley HB. Natural history of subarachnoid hemorrhage, intracranial aneurysms and arteriovenous malformations. J Neurosurg 1966;25:321–368
- Wiebers DO, Whisnant JP, O’Fallon WM. The natural history of unruptured intracranial aneurysms. N Engl J Med 1981;304:696–726
- Wiebers DO, Whisnant JP, Sundt TM Jr, O’Fallon WM. The significance of unruptured intracranial saccular aneurysms. J Neurosurg 1987;66:23–29
- Asari S, Ohmoto T. Natural history and risk factors of unruptured cerebral aneurysms. Clin Neurol Neurosurg 1993;95:205–214
- Taylor CL, Yuan Z, Selman WR, et al. Cerebral arterial aneurysm formation and rupture in 20,767 elderly patients: hypertension and other risk factors. J Neurosurg 1995;83:812–819
- Yasui N, Suzuki A, Nishimura H, et al. Long-term follow-up of unruptured intracranial aneurysms. Neurosurgery 1997;40:1155–1160
- The International Study of Unruptured Intracranial Aneurysms Investigators. Unruptured intracranial aneurysms: risk of rupture and risks of surgical intervention. N Engl J Med 1998;339:1725–1733
- Juvela S, Porras M, Poussa K. Natural history of unruptured intracranial aneurysms: probability of and risk factors for aneurysm rupture. J Neurosurg 2000;93:379–387
- Tsutsumi K, Ueki K, Morita A, et al. Risk of rupture from incidental cerebral aneurysms. J Neurosurg 2000;93:550–553
- The International Study of Unruptured Intracranial Aneurysms Investigators. Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet 2003;362:103–110
- Ujiie H, Tamano Y, Sasaki K, et al. Is the aspect ratio a reliable index for predicting the rupture of a saccular aneurysm? Neurosurgery 2001;48:495–503
- Weir B, Amidei C, Kongable G, et al. The aspect ratio (dome/neck) of ruptured and unruptured aneurysms. J Neurosurg 2003;99:447–451
- Winslow CM, Solomon DH, Chassin MR, Kosecoff J, Merrick NJ, Brook RH. The appropriateness of carotid endarterectomy. N Engl J Med 1988;318:721–727
- Barnett HJ, Plum F, Walton JN. Carotid endarterectomy: an expression of concern. Stroke 1984;15:941–943
- Warlow C. Carotid endarterectomy: does it work? Stroke 1984;15:1068–1076
- Huber TS, Durance PW, Kazmers A, Jacobs LA. Effect of the asymptomatic carotid atherosclerosis study on carotid endarterectomy in Veterans Affairs Medical Centers. Arch Surg 1997;132:1134–1139
- Hsia DC, Moscoe LM, Krushat WM. Epidemiology of carotid endarterectomy among medicare beneficiaries 1985–1996 update. Stroke 1998;29:346–350
- Tu, JV, Hannan EL, Anderson GM, et al. The fall and rise of carotid endarterectomy in the United States and Canada. N Engl J Med 1998;339:1441–1447
- Morasch MD. Carotid endarterectomy: characterization of recent increases in procedure rates. J Vasc Surg 2000;31:901–909
- Kresowik TF, Braztler D, Karp HR, et al. Multistate utilization, processes, and outcomes of carotid endarterectomy. J Vasc Surg 2001;33:227–235
- Feasby TE, Quan H, Ghali WA. Geographic variation in the rate of carotid endarterectomy in Canada. Stroke 2001;32:2417–2422
- North American Symptomatic Carotid Endarterectomy Trial Collaborators. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med 1991;325:445–453
- Barnett HJM, Taylor DW, Eliaziw M, et al, for the North American Symptomatic Carotid Endarterectomy Trial Collaborators. Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis. N Engl J Med 1998;339:1415–1425
- European Carotid Surgery Trialists’ Collaborative Group. MRC European Carotid Surgery Trial: interim results for symptomatic patients with severe (70–99%) or with mild (0–29%) carotid stenosis. Lancet 1991;337:1235–1243
- European Carotid Surgery Trial. Endarterectomy for moderate symptomatic carotid stenosis: interim results from the MRC European Carotid Surgery Trial. Lancet 1996;347:1591–1593
- Mayberg MR, Wilson SE, Yatsu F, et al. For the Veterans Affairs Cooperative Studies Program 309 Trialist Group. Carotid endarterectomy and prevention of cerebral ischemia in symptomatic carotid stenosis. JAMA 1991;266:3289–3294
- Rothwell PM, Eliasziw M, Gutnikow A, et al. Carotid Endarterectomy Trialists’ Collaboration: analysis of pooled data from the randomized controlled trials of endarterectomy for symptomatic carotid stenosis. Lancet 2003;361:107–116
- Fox AJ. How to measure carotid stenosis. Radiology 1993;186:316–318
- Barnett HJ, Meldrum HE, Eliasziw M, for the North American Symptomatic Carotid Endarterectomy Trial (NASCET) collaborators. The appropriate use of carotid endarterectomy. CMAJ 2002;166:1169–1179
- Kistler JP, Furie KL. Carotid endarterectomy revisited. N Engl J Med 2000;342:1743–1745
- Biller J, Feinberg WM, Castaldo JE, et al. Guidelines for carotid endarterectomy: a statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Association. Stroke 1998;29:554–562
- Gorelick PB, Sacco RL, Smith DB, et al. Prevention of a first stroke: a review of guidelines and a multidisciplinary consensus statement from the National Stroke Association. JAMA 1999;281:1112–1120
- Sacco RL. Clinical practice: extracranial carotid stenosis. N Engl J Med 2001;345(15):1113–1118
- Perry JR, Szalai JP, Norris JW, for the Canadian Stroke Consortium. Consensus against both endarterectomy and routine screening for asymptomatic carotid stenosis. Arch Neurol 1997;54:25–28
- Barnett HJ, Meldrum ME. Carotid endarterectomy: a neurotherapeutic advance. Arch Neurol 2000;57:40–45
- Moore WS, Barnett JHM, Beebe HG, et al. Guidelines for carotid endarterectomy: a multidisciplinary consensus statement from the Ad Hoc Committee, American Heart Association. Stroke 1995;26:188–201
- Findlay JM, Tucker WS, Ferguson GG, Holness RO, Wallace MC, Wong JH. Guidelines for the use of carotid endarterectomy: current recommendations from the Canadian Neurosurgical Society. CMAJ 1997;157:653–659
- Wolf PA, Clagett GP, Easton JD, et al. Preventing ischemic stroke in patients with prior stroke and transient ischemic attack: a statement from healthcare professionals from the Stroke Council of the American Heart Association. Stroke 1999;30:1991–1994
- Albers GW, Hart RG, Lutsep HL, Newell DW, Sacco RL. Supplement to the guidelines for the management of transient ischemic attacks: a statement from the Ad Hoc Committee on Guidelines for the Management of Transient Ischemic Attacks, Stroke Council, American Heart Association. Stroke 1999;30:2502–2511
- Benavente O, Eliaszw M, Streifler JY, Fox AJ, Barnett HJ, Meldrum H; North American Symptomatic Carotid Endarterectomy Trial Collaborators. Prognosis after transient monocular blindness associated with carotid artery stenosis. N Engl J Med 2001;345:1084–1090
- Morgenstern LB, Fox AJ, Sharpe BL, Eliasziw M, Barnett HJ, Grotta JC. The risks and benefits of carotid endarterectomy in patients with near occlusion of the carotid artery. Neurology 1997;48:911–915
- Rothwell PM, Gibson R, Warlow CP, on behalf of the European Carotid Trialists’ Collaborative Group. Interrelation between plaque surface morphology and degree of stenosis on carotid angiograms and the risk of ischemic stroke in patients with symptomatic carotid stenosis. Stroke 2000;31:615–621
- Eliasziw M, Streifler JY, Fox AJ, Hachinski VC, Ferguson GG, Barnett HJ. Significance of plaque ulceration in symptomatic patients with high-grade carotid stenosis. Stroke 1994;25:304–308
- Gasecki AP, Eliasziw M, Ferguson GG, Hachinski V, Barnett HJ. Long-term prognosis and effect of endarterectomy in patients with symptomatic severe carotid stenosis and contralateral carotid stenosis or occlusion: results from NASCET. J Neurosurg 1995;83:778–782
- Barnett HJM, Meldrum HE, Eliasziw M. For the North American Syptomatic Carotid Endantenectory Trial (NASCET) Collaborators. CMAJ 2002;166(9):1169–1179.
- Kappelle LJ, Eliasziw M, Fox AJ, Sharpe BL, Barnett HJ. Importance of intracranial atherosclerotic disease in patients with symptomatic stenosis of the internal carotid artery. Stroke 1999;30:282–286
- Henderson RD, Eliasziw M, Fox AJ, Rothwell PM, Barnett HJ. Angiographically defined collateral circulation and risk of stroke in patients with severe carotid artery stenosis. Stroke 2000;31:128–132
- Streifler JY, Eliasziw M, Benavente OR, et al, and the North American Symptomatic Carotid Endarterectomy Trial Group. Prognostic importance of leukoaraiosis in patients with symptomatic internal carotid artery stenosis. Stroke 2002;33:1651–1655
- The CASANOVA Study Group. Carotid surgery versus medical therapy in symptomatic carotid stenosis. Stroke 1991;22:1229–1235
- Mayo Asymptomatic Carotid Endarterectomy Study Group. Results of a randomized controlled trial of carotid endarterectomy for asymptomatic carotid stenosis. Mayo Clin Proc 1992;67:513–518
- Hobson RW, Weiss DG, Fields WS, et al, for the Veterans Affairs Cooperative Study Group. Efficacy of carotid endarterectomy for asymptomatic carotid stenosis. N Engl J Med 1993;328:221–227
- Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. Endarterectomy for asymptomatic carotid artery stenosis. JAMA 1995;273:1421–1428
- The European Carotid Surgery Trialists Collaborative Group. Risk of stroke in the distribution of an asymptomatic carotid artery. Lancet 1995;345:209–212
- Inzitari D, Eliasziw M, Gates P, et al. The causes and risk of stroke in patients with asymptomatic internal-carotid artery stenosis. N Engl J Med 2000;342:1693–1700
- Hougaku H, Matsumoto M, Handa N, et al. Asymptomatic carotid lesions and silent cerebral infarction. Stroke 1994;25:566–570
- Norris JW, Zhu CZ, Bornstein NM, Chambers BR. Vascular risks of asymptomatic carotid stenosis. Stroke 1991;22:1485–1490
- Moore WS, Boren C, Malone JM, et al. Natural history of nonstenotic, asymptomatic ulcerative lesions of the carotid artery. Arch Surg 1978;113:1352–1359
- Dixon S, Pais SO, Raivola C, et al. Natural history of nonstenotic, asymptomatic ulcerative lesions of the carotid artery: a further analysis. Arch Surg 1982;117:1493–1498
- Autret A, Pourcelot L, Saudea D, Marchal C, Bertrand P, de Boisvilliers S. Stroke risk in patients with carotid stenosis. Lancet 1987;1:888–890
- Wechsler LR. Ulceration and carotid artery disease. Stroke 1988;19:650–653
- Rutgers DR, Klijn CJM, Kappelle LJ, Eikelboom BC, van Huffelen AC, van der Grond J. Sustained bilateral hemodynamic benefit of contralateral carotid endarterectomy in patients with symptomatic internal carotid artery occlusion. Stroke 2001;32:728–734
- Liapis CD, Kakisis JD, Kostakis AG. Carotid stenosis: factors affecting symptomatology. Stroke 2001;32:2782–2786
- Golledge J, Greenhalgh RM, Davies AH. The symptomatic carotid plaque. Stroke 2000;31:774–781
- Bock RW, Grey-Weale AC, Mock PA, Robinson DA, Irwig L, Lusby RJ. The natural history of asymptomatic carotid artery disease. J Vasc Surg 1993;17:160–171
- Reilly LM, Lusby RJ, Hughes L, Ferrell LD, Stoney RJ, Ehrenfeld WK. Carotid plaque histology using real-time ultrasonography: clinical and therapeutic implications. Am J Surg 1983;146:188–193
- AbuRahma AF, Wulu JT, Crotty B. Carotid plaque ultrasonic heterogeneity and severity of stenosis. Stroke 2002;33:1772–1775
- Molloy J, Markus HS. Asymptomatic embolization predicts stroke and TIA risk for patients with carotid artery stenosis. Stroke 1999;30:1440–1443
- Karp HR, Flanders WD, Shipp CC, Taylor B, Martin D. Carotid endarterectomy among Medicare beneficiaries: a statewide evaluation of appropriateness and outcome. Stroke 1998;29:46–52
- Findlay JM, Marchak BE. Reoperation for acute hemispheric stroke after carotid endarterectomy: is there any value? Neurosurgery 2002;50:486–492
- Feasby TE. The appropriateness and effectiveness of stroke prevention. In: Hachinski, V, Norris, J, eds. Stroke Prevention. Oxford/New York: Oxford University Press; 2001:295–312
- Spetzler RF, Martin N, Hadley MN, Thompson RA, Wilkinson E, Raudzens PA. Microsurgical endarterectomy under barbiturate protection: a prospective study. J Neurosurg 1986;65:63–73
- Mayo SW, Eldrup-Jorgensen J, Lucas FL, Wennberg DE, Bredenberg CE. Carotid endarterectomy after NASCET and ACAS: a statewide study. North American Symptomatic Carotid Endarterectomy Trial. Asymptomatic Carotid Artery Stenosis Study. J Vasc Surg 1998;27:1017–1022
- Cebul RD, Snow RJ, Pine R, Hertzer NR, Norris DG. Indications outcomes, and provider volumes for carotid endarterectomy. JAMA 1998;279:1282–1287
- Kresowik TF, Hemann RA, Grund SL, et al. Improving the outcomes of carotid endarterectomy: results of a statewide quality improvement project. J Vasc Surg 2000;31:918–926
- Oliver SE, Thomson RG. Are variations in the use of carotid endarterectomy explained by population need? Astudy of health service utilization in two English health regions. Eur J Vasc Endovasc Surg 1999;17:501–506
- Ferris G, Roderick P, Smithies A, et al. An epidemiological needs assessment of carotid endarterectomy in an English health region: is the need being met? BMJ 1998;317(7156):447–451
- Findlay JM, Nykolyn L, Lubkey TB, Wong JH, Mouradian M, Senthilselvan A. Auditing carotid endarterectomy: a regional experience. Can J Neurol Sci 2002;29:326–332
- Cote R, Battista RN, Wolfson C, Boucher J, Adam J, Hachinski VC. The Canadian Neurological Scale: validation and reliability assessment. Neurology 1989;39:638–643
< div class='tao-gold-member'>