15 | Cerivcal Epidural Abscess |
 | Case Presentation |
History and Physical Examination
A 56-year-old male presented to a university hospital complaining of neck pain and severe burning pain down his left upper extremity. The patient reported that 3 weeks prior to presentation, he had tripped and fallen approximately 4 feet, striking the left side of his body. Since then, he had experienced left upper extremity numbness and pain, but he chose not to seek medical attention. On the morning of his presentation the patient fell a second time, again injuring his left side. The fall exacerbated his symptoms, and he complained of severe burning pain radiating down his left arm and numbness in both of his legs.
Detailed past medical, past surgical, social, and family history was obtained and revealed that the patient had hypertension and type 2 diabetes mellitus. He admitted to smoking a pack of cigarettes daily and to occasional alcohol consumption but denied any illicit drug use. On review of systems, the patient noted feeling run down, having a general sense of malaise. He denied any fevers, chills, night sweats, or recent illnesses. Extended review failed to reveal any further pertinent information. The patient had a low-grade fever of 100.6°F. Vital signs were stable. A thorough neurological exam was performed and revealed the patient to have 5/5 motor strength with the exception of the proximal muscles of the left upper extremity, which were limited secondary to his severe pain. He had no abnormal reflexes or any evidence of a myelopathy. He did note paresthesias in his left arm and a Lhermitte type of shooting pain sensation upon extension of his neck. Initial laboratory studies showed the patient to have no hematologic abnormalities. His leukocyte count was within the normal range at 6.9 K. Erythrocyte sedimentation rate (ESR) checked shortly after admission was elevated at 50 mm/h.
Radiological Findings
Plain radiographs were negative for fracture or malalignment. Computed tomography demonstrated linear lucencies in the left facet joints at C2-3 and C4-5, felt to represent old fractures with subsequent osteophyte formation. No acute fractures were seen, and normal cervical alignment and lordotic curvature were maintained. Posterior osteophytosis was seen at the C2-3, C3-4, and C4-5 levels but no significant central canal stenosis was appreciated.
Arrangements were made for magnetic resonance imaging (MRI) of the cervical spine, and this was obtained later that day. The MRI revealed an epidural process posterior to the spinal cord that was hyperintense on T2-weighted images and isointense on T1. The process enhanced greatly on T1 postgadolinium images (Fig. 15–1A,B). The collection extended from C3 to upper thoracic spinal levels and could be seen displacing the cord anteriorly and to the right. The lesion appeared to be contiguous with an enhancing collection located within the paraspinal musculature and soft tissue of the neck by means of the left neural foramina. The appearance of this collection was felt to be most consistent with an epidural spinal abscess of the cervical spine. The MRI failed to show a significant component of osteomyelitis or diskitis.
Diagnosis
Cervical epidural abscess
 | Background |
Spinal epidural abscess is a relatively rare condition. The incidence of this potentially devastating infection appears to have increased in recent years. Current best estimates of incidence in the United States is two hospitalizations per 10,000,1 higher than original estimates of two to 25 patients per 100,000.2,3 This apparent increase, however, may be a reflection of advances in diagnostic imaging and greater recognition of the disease process as opposed to a true increase in prevalence. The disease and its treatment were described in the literature by Taylor and Kennedy in 1923 followed soon thereafter by Dandy in 1926.4,5 Since that time the treatment of choice consisting of urgent surgery and decompression of the lesion has remained nearly universal.
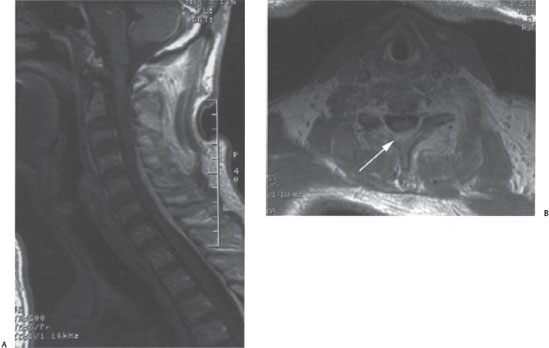
Figure 15–1 (A) T1 sagittal and (B) axial images demonstrating brightly enhancing cervical epidural abscess extending (arrow) caudally from C4 to the upper thoracic spine.
Location in the spine varies in incidence, with infection most commonly occurring in lumbar (39.3%), followed by thoracic (33.3%), and cervical (27.2%) levels.6 However, the rate of epidural abscess manifested as a complication of spondylodiskitis was found to be 90% in the cervical spine, as opposed to 33.3% in the thoracic, and 23.6% in the lumbar spine. This suggests that, although osteomyelitis occurs less frequently in the cervical region, the propensity to develop neurological compromise as a result of epidural abscess is significantly higher.3
Spinal epidural abscess occurs most commonly in males over the age of 30, with the majority of patients being in their sixties. The male:female ratio in epidural abscess is 2.5:1.7 Epidural infection in the pediatric population is virtually unheard of, although the rare case reports exist.8 The most commonly identified risk factor for epidural abscess is illicit intravenous drug use (27% of cases).1 Nonspinal infections can lead to epidural abscess either by hematogenous spread or by direct extension. Cellulitis can lead to infection seeded by the blood, whereas retropharyngeal abscess, often after surgery (21% of cases), can extend directly posteriorly, leading to osteomyelitis and epidural abscess. Diabetes mellitus is accepted as an important risk factor for the development of infection and is identified in ~20% of cases. Disease states and therapies that lead to an immunocompromised state such as human immunodeficiency virus, malignancy, and chronic steroid use also predispose patients to the development of abscess in the spinal column.1 Hemodialysis and indwelling catheters (which often become infected) can predispose patients with end-stage renal disease to the formation of epidural abscesses, often with methicillin-resistant Staphylococcus aureus.9–11
Abscess formation after epidural steroid injection has been described but its incidence is very low, estimated at one case per 400,00012. More commonly epidural abscess can develop as a result of infection of indwelling catheters such as those used in epidural anesthesia.7 Recent spinal trauma has also been identified as a risk factor for the development of epidural infection. It is theorized that blunt trauma leads to the formation of a locus minoris resistentiae, facilitating the implantation of infection by a hematogenous route.7
The classic clinical features that accompany cervical epidural abscess are neck pain, fever, and some degree of neurological dysfunction. Pain is the predominant feature in most cases and can be either axial or radicular in nature, depending on the location of the infection within the spinal canal. Fever, defined as temperature > 101°F, is a presenting sign ~50% of the time and is clearly not required for diagnosis. Neurological dysfunction such as weakness, sensory loss, and bowel or bladder dysfunction is an often-devastating sequela of epidural abscess, and its presence and degree help dictate management.
Laboratory studies useful in the diagnosis are complete blood count with differential, ESR, and C-reactive protein (CRP). In addition, routine blood cultures should be obtained to help identify a causative organism. Leukocytosis with a moderate elevation in white blood count (WBC) > 15,000/mm1 often accompanies spinal epidural abscess, but a normal WBC is not uncommon. Again, these patients can be immunocompromised and thus are unable to mount an appropriate immune response with the development of leukocytosis and fever. ESR has been shown recently to be a very useful test to help raise the suspicion and confirm the diagnosis of epidural abscess. In several reports ESR has shown to be consistently elevated (95%) in cervical epidural abscess.13 Elevation greater than 30 mm/h is common even in the absence of fever and leukocytosis.14 Elevations greater than 100 mm/h in a patient with neck pain is highly suggestive of epidural infection.15 CRP is often elevated and can support the diagnosis as well as gauge response to treatment.
Blood cultures are positive in one half to two thirds of cases. Blood and abscess cultures have been shown to be 100% concordant when both are positive and thus are very helpful in focusing antibiotic therapy.1 The causative organism is most often Staphylococcus aureus (63% of cases). Species are usually methicillin-sensitive S. aureus (MSSA), however, cases of methicillin-resistant S. aureus (MRSA) do occur. Other less common causative bacteria are Streptococcus species, Pseudomonas, Escherichia coli, and Lactobacillus. Mixed flora with combinations of the above organisms also occurs, as well as infection with oral flora such as Prevotella oris and Peptostreptococcus micros.1,3,7,16,17
Radiographic evaluation with MRI is the diagnostic test of choice for spinal epidural abscess. MRI with and without gadolinium contrast should be obtained in a timely fashion if the diagnosis of epidural infection is suspected. Nonenhanced T1 -weighted images will show an epidural collection that is heterogeneous and isointense or hypointense compared with the adjacent dura and spinal cord. T2-weighted images will show the collection in the epidural space to be hypointense. The T1 postcontrast series is the most useful in confirming the diagnosis. The images will demonstrate a greatly enhancing heterogeneous collection in the epidural space. For patients in whom an MRI cannot be obtained, a computed tomographic (CT) myelogram can demonstrate the lesion but carries with it the added risks of myelography, including the risk of seeding infection into the subarachnoid space if concomitant lumbar epidural abscess exists. MRI with contrast and CT myelogram has been shown to have equivalent sensitivities (91 to 92%) in detecting epidural abscess.1
Curry described three main goals in therapy for epidural abscess: (1) preservation of normal neurological function, (2) prevention of worsening of existing neurological deficits, and (3) optimization for improvement and return of function.1,18 Treatment options consist of decompression and evacuation of the abscess followed by a course of antibiotics versus antibiotics alone. Urgent surgery remains the treatment of choice; however, some have had success with conservative therapy1,17,19–21 as well as with percutaneous procedures.22,23
Most spine surgeons agree that surgery for epidural abscess of the cervical spine should be undertaken as soon as the diagnosis is confirmed and the patient is cleared medically. This feeling has remained constant since the disease process was described in the 1920s and is well supported in the literature.6,18,21,24–30 Surgery has several advantages over conventional therapy (i.e., antibiotics alone), in that it allows for rapid decompression of neural structures as well as adequate sampling of the collection for culture. Once a causative organism is identified, treatment continues postoperatively with IV antibiotics tailored to the bacterium’s susceptibility, usually for 4 to 6 weeks. If osteomyelitis is also involved, IV antibiotics are typically continued for 8 weeks. Some surgeons advocate continued therapy with oral antibiotics 2 to 4 weeks beyond this period.18
 | Authors’ Preferred Method of Surgical Treatment |
The patient in the clinical vignette underwent emergent posterior decompression of the cervical spine and evacuation of the epidural collection. Formal laminectomies, with care taken to avoid violating the facets, were preformed from the inferior edge of C4 through the leading edge of C7 allowing for decompression of the abscess. Thick granulomatous material was encountered and was carefully washed free from the epidural space with copious irrigation. The superior and inferior margins of the laminectomies were inspected at the conclusion of the case and felt to be free of any residual epidural material. The epidural collection was sent to the microbiology laboratory for Gram stain and culture.
In most cases of cervical epidural abscess, we prefer posterior decompression with laminectomy, although decompression with less bony disruption has been achieved using either laminotomies or hemilaminectomies. Additionally, selected cases may also benefit from supplemental posterior instrumentation and fusion if instability is present. The anterior approach is reserved for those cases in which the infection is predominantly anterior to the cord and for which diskitis and osteomyelitis are a major component.
Pearls and Pitfalls
The posterior approach classically involves laminectomy and evacuation of the abscess. If the infection is acute (< 12 to 16 days) copious amounts of frank pus are often encountered, and this obviously should be sent for Gram stain and culture including aerobic, anaerobic, fungal, and mycobacterium, Older collections often have a more granulation tissue-like consistency, which can be tightly adherent to the dura. Care must be taken when attempting to remove these lesions so as to avoid a dural tear with a resultant spinal fluid leak. Dural tear in the face of epidural infection carries with it a high risk of meningitis. In instances where the lesion involves multiple spinal levels some have advocated the use of irrigation devices to be passed underneath the lamina so as to avoid excessive bony disruption. In a similar fashion, if the lesion is acute (i.e., pus) and involves multiple levels the judicious use of laminotomies and catheters for evacuation of the collection and irrigation has had some success.23 These techniques should only be attempted in cases where there is enough room between the dura and the lamina to safely place the catheter. In all cases, regardless of techniques, copious amounts of irrigation should be used and drains should be left in place postoperatively.



