Wavelength (nm) | Lasertype | α (cm-1) | L (cm) |
|---|---|---|---|
980 | Diode | 0.473 | 2.11 |
1064 | Nd:YAG | 0.144 | 6.94 |
2120 | Ho:YAG | 36.40 | 0.028 |
The penetration depth given in the table is calculated for water only and is different in the disc material conditionally due to scattering etc. Gangi et al. evaluated the tissue penetration depth for Nd:YAG of nearly 7 mm [14]. Ho:YAG Laser shows a relatively lower penetration depth compared to the Diode Laser, followed by the Nd:YAG Laser.
In addition to the Laser and the tissue type, one has to take more parameters into account in order to understand the tissue interactions (Fig. 6.2).
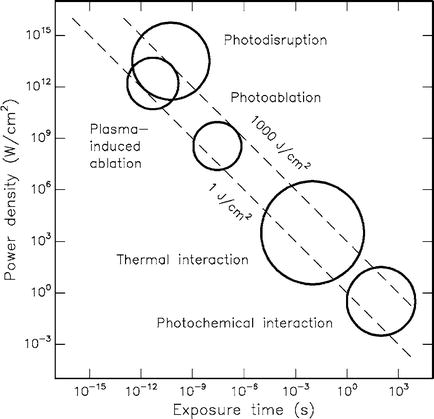

Fig. 6.2
Laser- tissue interaction – circles show a rough calculation (With permission from Niemz [15])
Laser power, pulse energy, pulse duration, fiber diameter, energy and power density and the duration of the application etc., are important parameters showing a significant influence on the processes taking place in the target tissue [4, 9, 15–17].
Short, high energetic pulses with a high power density lead to tissue disruption and ablation effects. In contrast, longer laser pulses with lower power densities lead to thermal effects .
Ho:YAG Laser have the highest power density and show ablative reactions and lower thermal effects [6]. The disadvantage of the short pulses is the cavitation wave, with possible negative effects on the adjacent tissue [14, 18].
Nd:YAG have a deeper tissue penetration and longer pulse duration, therefore the thermal effects are greater in the tissue [6, 19].
Experiments by Laser und Medizintechnologie GmbH Berlin show [20], that the tissue penetration of the diode laser (980 nm) when compared to the Nd:YAG Laser, is reduced by half. The reason for this is mainly the high water content of the disc material. But the ablation effects of the Ho:YAG are not attained at this power density.
One has to take into account that carbonization of the fiber tip leads to changed optical properties. Schlangmann et al. examined [21] the carbonization effect of the Nd:YAG Laser. If the fiber tip is carbonized, a direct vaporization of tissue is possible.
Discussions with those who employ it already showed that the hot tip technique is often used by directing the fiber tip to a wooden spatula in order to carbonize the fiber tip.
This technique should avoid a too high penetration depth and possible damage to vascular tissue because of a high absorption of hemoglobin [19, 20].
It is not recommended because it changes the array of the laser probe to an unpredictable amount.
On the contrary a 980 nm Diode laser with a lower tissue penetration should be selected.
The following figures show the thermal properties of a Diode laser in a thin Agar-Agar layer demonstrating the tissue penetration and the heat conduction (Fig. 6.3).
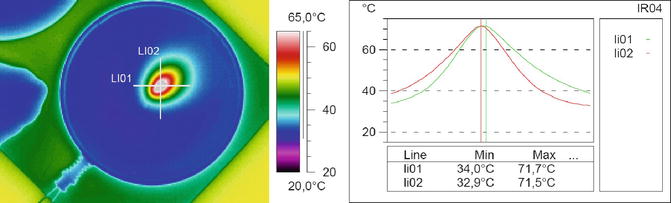

Fig. 6.3
Temperature profile in Agar-Agar for demonstration of the tissue penetration
The fibre is symbolized. The cross shows the measuring line of the temperature profile in the diagram.
Parameter: λ = 980 nm, P = 7.5 W, Pon = 750 ms, Poff = 750 ms
Mode of Action of the PLDD
Vaporisation
Based on the described tissue interaction between laser radiation and the tissue, one can assume that the tissue will be vaporized leading to a volume reduction [1, 8, 21–24].
The quantitative evaluation of this vaporization effect was done as early as 1995/1996. Buchelt et al. [22] describe a nearly linear relationship between resection rate and applied energy (for the Ho:YAG Lasers), but no significant increase in the ablation rate and maximal power between 10 and 32 W.
Similar results are found by Schlangmann et al. according to ablation rate and applied power [21]. Furthermore, the Ho:YAG and Nd:YAG lasers were compared. The ablation rate was 200 mg (1064 nm, Pmax = 20 W, Pon = 1 s, Poff = 5 s, E = 1500 J).
With the Ho:YAG Laser, Min et al. [8] examined an ablation rate of 1.89 g following the application of 20,000 J.
Shrinkage
In addition to the vaporization effects, tissue shrinkage of collagen takes place by thermal exposure.
In 1995 Siebert in [25], Choy and Altman in [23] and 1999 Hellinger in [26] were able to demonstrate this effect. Several other researchers confirmed tissue shrinkage of disc material [27–29]. Wang et al. showed that a temperature of 75 °C is sufficient, to result in collagen shrinkage. A higher temperature showed no additional effects.
The Diode- and the Nd:YAG Laser seem to be superior to the Ho:YAG, because these two systems show a deeper thermal effect on the tissue and an increased surface where these effects show an influence .
Decompression
The two previously described effects, vaporization and shrinkage of the collagen, lead to a reduction of volume and a lowering of the intradiscal pressure.
Choy considered the disc to be a hydraulic system in which small changes in volume could lead to relatively big changes in the tissue pressure [23].
Hellinger and Stern [30] describe the shrinking effect to be as high as 14 %, employing a Nd:YAG Laser.
According to the property of nucleus pulposus to accumulate water the, shrinking of the disc tissue could create a more permanent effect in relation to its vaporization, because it can be expected, that in course of time the nucleus will cumulate more volume [31].
It is generally accepted that the intradiscal pressure plays an important role in the pathophysiology of pain. During discographies radicular pain can be provoked by an injection of contrast media and an increase of the intradiscal pressure.
In addition to the elevation of the intradiscal pressure and the provocation of pain a radiological examination can be carried out. Discography can be performed in the cervical spine as well as in the rest of the spinal column [7].
Pathological discs show the so-called dicogenic pain, at relatively low intradiscal pressures. (discpain), healthy discs, tolerate higher intradiscal pressures [32]. These conclusions are only correct when the annulus fibrosus is intact. All degenerative changes that lead to a sequestrated disc herniation or a disrupted annulus fibrosus are a contraindication for PLDD .
Denervation
The observation that an increase of the intradiscal pressure can lead to pain, demonstrates that it is possible that the disc in itself can be painful.
Several studies show a neuronal innervation of the disc tissue. Inman et al. were the first to show this in 1947 [33]. These observations are not generally accepted. In the subsequent years several studies were able to demonstrate an innervation of the disc tissue. Most of these studies were performed on lumbar disc material [32, 34], however a few studies could show mechanoreceptors and neuronal tissue in cervical disc material [7, 35].
Annular fissures and micro fractures may lead to a deep penetration of the nerve tissue into the degenerated annulus fibrosus, resulting in increased pain during movements in the motion segment [36].
The laser could lead to a denervation of the disc material and therefore a decreased pain perception.
It is therefore recommended that the laser fiber should not be placed into the center of the disc during a lumbar laser treatment. The probe should be placed at the border of the annulus fibrosus [37, 38].
Because of the different, more anterolateral approach in the treatment of cervical disc pathologies, the posterior part of the disc can be easily reached, but it is recommended avoiding a posterior placement of the probe, in order to prevent damage of neuronal structures. Due to the tissue penetration of the laser radiation, a lesion of neuronal structures cannot be excluded .
Anti Inflammatory Effects
In addition to the innervation of degenerated disc material, biochemical processes like the induction of inflammatory reactions in the disc material can lead to the generation of pain.
Phospholipase A2, Interleukin-1 and nitrogen oxides are found in degenerated disc material. This could lead to the synthesis of the neuropeptide Substance P and to the induction of the pain cascade.
During the laser application a denaturation of these cytokines is assumed [39].
Summary of Action
As opposed to alternative treatment options for discogenic pain like the IDET, PLDD shows more modes of action. PLDD acts not solely anti-inflammatory and via a denervation but results in a shrinkage and volume reduction of the disc material. These effects explain the rapid improvement of pain syndromes that most of the patients describe after PLDD.
Selection of Parameters for Cervical Treatment
In the literature there is a great variety of recommended parameters. Especially the applied energy and the maximal power are inhomogenously reported. Furthermore some peers do not give any information about the selected parameters at all.
Looking especially at cervical laser applications one notices that Hellinger et al. performed as early as 1990 first laser applications for cervical pain syndromes [40] However the cervical treatment amounts to only 7–8 % of all laser applications in the human spine.
In the following years there is no marked increase in the amount of cervical treatments. Choy and Hellinger et al. [41] repost more than 22,615 PLDD treatments of which 1641 were performed on the cervical spine. These numbers refer to their own patients and to a literature review up to 2009. Our own data confirm the previously reported amount of 8 % of cervical treatments.
In the following table some parameters are given for laser treatment in the cervical spine
Laser | Pmax (W) | Pulson (s) | Pulsoff (s) | Peff (W) | Emax (J) | Year |
|---|---|---|---|---|---|---|
Nd:YAG | 10 | 1 | 3 | 2.5 | 300 | [42] 2004 |
Nd:YAG | 10–12 | 0.2–1.0 | 0.5–1.7 | 2.9–4.4 | 300–800 | [7] 2011 |
Nd:YAG | 10 | 0.3 | 1.7 | 1.5 | 120–500 | [43] 2001 |
Nd:YAG | 15–20 | 0.3 | – | – | 350–450 | [26] 1999 |
Nd:YAG | 5/10/20 | – | – | – | 400–700 | [44]a 2004 |
810 nm | 15 | 1.0 | 2.0 | 5 | 300–1000 | [45] 2007 |
Ho:YAG | 12 | 0.5 J/12 Hz + pause | 6 | 600–800 | [46] 2001 | |
Ho:YAG | 2.5 | 0.5 J/5 Hz | 2.5 | 400 | [25] 1995 | |
KTP532 | 12 | 0.2 | 0.5 | 3.4 | 600–800 | [46] 2001 |
980 nm | 7.5 | 0.7 | 1.0 | 3.1 | 180–200 | Authors |
When comparing lumbar laser treatments with cervical laser applications, it becomes evident that for the lumbar treatment, significantly higher parameters were chosen.
Siebert et al. report [47] in in-vitro experiments thermal consequences of the Nd:YAG Lasers employing a power up to 40 W, a pulse duration of 1 s and a total applied power of 1000 J. Min et al. present data of PLDD treatments with Ho:YAG 14 W and a total applied power of up to 20,000 J.
In the early years of lumbar laser application , the parameters differed widely. Due to a vast amount of subsequent in-vivo und in-vitro experiments parameters could be optimized [21, 22, 27, 38, 39, 44, 47–50].
If devices are used which show a longer pulse duration (980 nm Diode laser, Nd:YAG Laser), one could use different pulse duration and pulse pause as well as the pulse power. These observations lead to a continuous decrease in the laser power. At present the recommended parameters for lumbar and cervical laser applications are nearly similar. The only exception is that the applied total power is significantly lower because the total volume of a cervical disc is significantly lower. Therefore 35–50 % of the recommended total power of lumbar applications is employed for cervical treatments [14, 26, 43, 51, 52].
Summarizing the literature it becomes evident that due to the vast amount of possible parameters and the biological properties of the disc material itself a highly complex situation results which makes it impossible to give standard recommendations for the PLDD treatment.
The total applied power is described as the primary parameter in several publications, but it is very important to describe how the total power is archived.
A high pulse power applied for a short amount of time result in the same total power as low pulse power applied for a longer period of time. The resulting tissue interaction however is most likely to be different [31].
In retrospect, however, there is a standardized recommendation of parameters with a certain amount of variation.
Looking at the wavelength the Nd:YAG Laser seems to be superior to the other systems, as well as the Diode laser, which was introduced for clinical applications in the human spine in 1990. A literature review by Chambers et al. [53] in 1995 reveals that 231 patients 90 % were treated by Nd:YAG and the rest of 23 patients were treated with Ho:YAG Laser devices.
Some surgeons still use Ho:YAG Laser systems . Yet looking at the physical parameters, it becomes, in our opinion, self -evident to employ Nd:YAG or diode laser systems.
The primary reason for the Ho:YAG Lasers was the attempt to increase the ablation rate by vaporization of tissue without significant thermal changes in the adjacent tissue. But it was demonstrated that a significant increase of the temperature in some areas is present when employing a Ho:YAG Laser system [54].
Later examinations revealed that a broad thermal effect plays an important role in the treatment of degenerative disc disease (shrinking, anti-inflammatory effects and denervation). Therefore it is recommended to choose a wavelength closer to the near infrared spectrum in order to archive a broader thermal effect.
It is clear that the Ho:YAG shows thermal effects. However, these effects occur as high temperatures at the distal end of the laser fiber without significant thermal penetration into the tissue. Because of the better thermal penetration of lasers, having a wavelength between 980 and 1064 nm, a larger area of disc tissue can be treated.
Reviewing the literature there is no publication concerning the use of the diode laser 980 nm for cervical pathologies. General observations for the intradiscal use of the diode laser can be found by the LMTB Berlin 1995 [20], 1998 Paul and Hellinger in [55] and Grönemeyer, Gervagez et al. since 2000 in [52, 56].
Our group Schneiderhan, Sommer et al. have been employing the 980 nm Laser since 2002 for the PLDD lumbar pathologies, and since 2004 for the cervical treatment. We had reliably good results in 3978 cases, of which 330 procedures were on the cervical spine.
Necessary Equipment
In order to perform the PLDD in a low complication setting the usual sterile drapes and an appropriate procedure room are necessary. Furthermore, the presence of an anaesthesiologist is mandatory for the cervical PLDD treatment.
Listed is the necessary equipment for the cervical PLDD
Radiology equipment (Fluroscopy or CT scanner) (alternatively MRI [28, 57] if MRI compatible cannulas are available and the laser fiber is long enough)
Basic drapes for a sterile work space
Contrast media for intradiscal application
Optional single-shot antibiotic treatment (Cephalosporin)
Diode laser 980 nm, 10 W maximal power (Fig. 6.4)
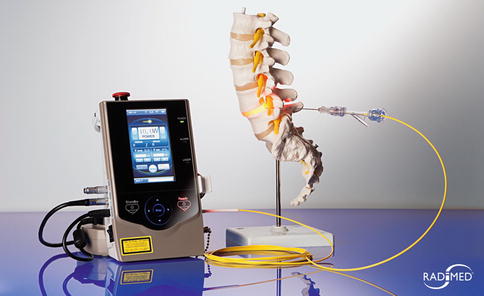
Fig. 6.4
Diode laser 980 nm and laser fiber set, Radimed GmbH
Single use laser set – cannula 21G and a laser fiber 360 μm as well as a fixation device. This device is especially important to prevent the laser fiber from sliding back into the cannula where it could lead to a marked increase of the temperature in the cannula with a possible detrimental effect on the adjacent tissue (Fig. 6.5).