and Róbert Bódizs2
(1)
Institute of Experimental Medicine and Institute of Neuroscience, Budapest, Hungary
(2)
Semmelweis University Institute of Behavioral Science, Budapest, Hungary
Abstract
The homeostatic sleep regulation idea underwent important development. Sleep homeostasis was first connected to the duration of the preceding awake time. Due to increasing innovative research in this field with convincing evidences on local sleep regulation, it seems that beyond the length of waking time, use-dependent afferent stimulation and synaptic upscaling (learning) are the main factors regulating the NREM sleep slow-wave activity (SWA). Further achievement of the same research line was to obtain evidences that plastic modulation of local slow-wave power during NREM sleep is closely related to the recreation of cognitive functions in the cortex, mainly in the frontal lobes. Slow-wave homeostasis and use-dependent plasticity are probably two sides of the same coin representing the biological function of slow-wave sleep.
Keywords
Sleep homeostasisUse-dependent plasticityLocal sleepSlow-wave activityDelta activity
Sleep is the price we have to pay for plasticity (Tononi and Cirelli 2003).
5.1 Discovery and First Views on Homeostatic Regulation
Homeostatic regulation in general is the control and maintenance of a stable and constant condition, achieved usually by negative feedback mechanism. One of the most powerful controls of the NREM sleep process is the homeostatic regulation expressed by the slow-wave components of sleep EEG. It is a common experience that sleep loss leads to increased sleep propensity and sleep after sleep deprivation lasts longer. Sleep loss is associated with impairment of cognitive functions and the subjective feeling of “sleepiness.” It is questionable if cognitive impairment would be the consequence of lack of sleep or it is due to the sleep-inducing mechanism mobilized as a homeostatic response.
By recording a particularly high number of whole night sleep records, Webb and Agnew (1971) was the first who has unequivocally shown that the time spent in stage 4 sleep is related to the duration of presleep wakefulness. Increased time spent awake was followed by increases in stage 4 sleep time. On the other hand, it was evident that the time spent in stage 4 sleep decreases during the sleep process. Thus, a wake-dependent increase and a sleep-dependent decrease of stage 4 sleep were evidenced, suggestive for a homeostatic regulation of the SWA, which is one of the main defining features of this sleep stage. The sleep-dependent decrease of slow waves was quantified and characterized by Feinberg et al. (1978) using the period amplitude analysis measures. This investigation is also a forerunner of the concept of homeostatic regulation of sleep SWA.
A group of sleep researchers and mathematicians in Zurich led by Alexander A. Borbély has clearly proven in 1980s that the intensity of NREM sleep as measured by the power spectra of SWA depends on the duration of the previous awake state (Borbély et al. 1981). In nap studies, a gradual increase was shown in the SWA in consecutive naps during the day (Dijk et al. 1987a). This increase in slow waves of naps was the mirror image of the exponential decline of SWA during sleep, e.g., sleep propensity increases exponentially during the waking time following a slow saturating function, while during sleep, it decays exponentially (Borbély 1982; Borbély et al. 1989), reflecting the homeostatic dynamism (Dijk et al. 1987b; Fig. 5.1).
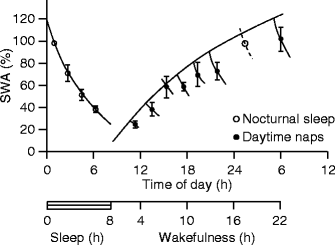

Fig. 5.1
Homeostatic regulation of sleep slow-wave EEG activity (0.75–4.5 Hz). Power of sleep slow-wave activity follows an exponentially decreasing trend during nocturnal sleep. On the other hand, daytime nap studies reveal the slowly saturating exponential function describing the increase of slow-wave activity in the absence of sleep. SWA slow-wave activity (Dijk et al. 1987a)
If a human being is sleep-deprived, the very next sleep will ensure the supplementation of the lost sleep. The substrate of this homeostasis was called by Borbély as “Process S,” and the power spectrum of the EEG slow-wave range 0.75–4.5 Hz proved to be a good quantitative measure of it. It was shown that the patterns of sleep EEG spectral alterations induced by sleep deprivation are characterized by increased SWA (and decreased spindling). This pattern is mirrored during successive sleep cycles of undisturbed sleep. This suggests that the dissipation of sleep need during the night sleep can reliably be measured by assessing SWA (Borbély et al. 1981). Moreover, because EEG slow-wave activity is the main defining feature of slow-wave sleep, the wake-dependent exponential increase and sleep-dependent exponential decline of SWA are reliably reflected in the minutes of slow-wave sleep (Knowles et al. 1986).
The exponential increase of sleep propensity during sleep deprivation was shown to be followed by an increase in the 0.5–2-Hz slow-wave incidence during recovery sleep. These slow waves of recovery sleep were of increased frequency when compared to the ones of baseline sleep (Bersagliere and Achermann 2010). The decline of delta activity in sleep was proved to be coupled with decreased incidence of high amplitude slow waves, a decreased slope of the individual slow waves, and an increased number of multipeak waves (Riedner et al. 2007).
McCarley (2007) have shown that adenosine acting in the basal forebrain is a putative mediator of homeostatic control. Increased need for sleep was accompanied by increased release of adenosine. In other experiments, injection of adenosine or an adenosine A1 receptor agonist into the rat basal forebrain or cat VLPO promoted sleep by inhibiting wake-promoting regions (Scammell et al. 2000; Strecker et al. 2000). The relationship between adenosine and sleep homeostasis is evident from the studies investigating the effects of naturally occurring functional polymorphisms of the gene coding the adenosine deaminase (ADA) enzyme in humans. The heterozygous G/A variant of the 22 G > A polymorphism of the gene encoding ADA results in reduced adenosine breakdown and consequent increases in the nightly amounts of slow-wave sleep and slow-wave EEG activity during both NREM and REM sleep (Rétey et al. 2005). Although similar results were revealed in studies on rats, it is premature to conclude that adenosine is the only and main neurochemical substrate of sleep homeostasis. However, as adenosine is a result of the breakdown of ATP, it is evident that the brain’s energy balance is involved in the homeostatic regulation of the sleep process. Other neurochemical factors involved in sleep homeostasis are the somnogenic cytokines (interleukin-1 beta [IL1-β] and tumor necrosis factor alpha [TNF-α]) as well as the molecules involved in long-term potentiation (LTP), like the brain-derived neurotrophic factor (BDNF).
The main aspect was the amount of prior wakefulness, considered by Wilse B. Webb and Alexander A. Borbély. This finding was coherent with the hypnotoxin theory of Henri Pieron (1913), suggesting that a wake-dependent release of some somnogenic chemical agent accounts for the accumulation of sleep need.
5.2 Use-Dependent Homeostatic Regulation and Local Plasticity: Sleep-Dependent Improvement of Learning and Plasticity
In the last 10–15 years, local aspects of homeostatic regulation have received more and more attention: the frontal preponderance of sleep slow-wave activity and frontal dominance of the recovery increase after sleep deprivation and dominant hemisphere preponderance (Achermann et al. 2001) were emphasized in several studies (Finelli et al. 2001; Cajochen et al. 1999; Marzano et al. 2010). This has been interpreted as evidence for the role of slow-wave sleep in human frontal cognitive functions (Horne 1993). Later, when local aspects of sleep regulation were increasingly evident, the concept of wake dependency was slowly completed with the notion of use dependency, which is in fact based on the amount of afferent inputs to a certain neural network. That is, equal amounts of wakefulness were shown to be followed by topographically different increases in SWA. Differences were attributed to the differences of use of the neural systems involved in processing the afferent inputs during wakefulness. Use dependency is in part a heritage of the infinite search for the hypnotoxin proposed by Pieron or the S-factor of Pappenheimer et al. (1975). The attempts to characterize this chemical agent have lead to the discovery of the strong link between sleep and the immune system. The slow-wave sleep-inducing effects of some somnogenic cytokines, like the TNF-α, were shown to be local. Local applications lead to increases in local sleep SWA (Yoshida et al. 2004).
Besides the frontal lobes involved in homeostatic regulation related to certain cognitive functions, other different regions producing use-dependent increase of SWA have been registered after specific use. Extensive sensory stimulation of one hand before sleep led to an increase of sleep delta power in the opposite hemisphere over the somatosensory arm area (Kattler et al. 1994). An opposite intervention, immobilization of the arm, caused a local reduction of delta power in the same localization (Huber et al. 2006). Similar results were found in rats after cutting their whiskers on one side and analyzing the changes in hemispheric asymmetry in their sleep SWA (Vyazovskiy et al. 2000).
Further aspects of local sleep concept came from the work of Krueger and Obál (1993), who showed indirect and direct evidences indicating that cortical columns oscillate between functional states defined by changing input-output relationship tested by amplitudes of evoked responses (Rector et al. 2009). Mosaics of sleeping columns can be found while other columns are continuously awake. The longer the column is in awake-like state, the higher the probability that it will turn to sleep. The probability to find sleeping columns also depends on the amount of afferent activity or on the neuronal traffic resulting in learning. Krueger and Obál (1993) emphasized that sleep is a statistical phenomenon: e.g., a sum of the local sleep processes leads to global/behavioral sleep if there is a sufficiently high number of neural networks involved. Krueger et al. (2008) suggest that global coordination of NREM is not due to a single sleep generator, but may reflect an emergent property of loosely coupled local processes.
Local sleep regulation is a crucial point in understanding the nature of sleep homeostasis. The original two-process model and its later specifications (Borbély 1982; Borbély et al. 1989) focused on global aspects of the process only. However, it is evident from the above-cited studies that local differences are significant and robust enough for considering their involvement in the global aspect of sleep regulation or sleep need. According to this view, sleep regulation centers are “just” coordinators, providing a more or less synchronized entry of many different networks into the sleep state. Sleep-inducing centers, like the VLPO region, are not per se sleep inducers, but synchronizers of many local sleep needs. Another view is that of Zavada, who changed the original two-process model in order to cover the phenomena of local sleep intensities (Zavada et al. 2009). The Process Z would be the one which is involved in local sleep regulation. In this view, there is a global sleep need (quantified by the Process S), which is independent from the local fluctuations of sleep intensity (Process Z).
Later, learning and synaptic plasticity was shown to be related to sleep SWA, and the notion of experience dependency emerged, suggesting that it is the net change in synaptic strength that is important for sleep homeostasis (Tononi and Cirelli 2003). Experience dependency means that equal amounts of wakefulness and afferent stimulation could lead to different rates of increase in sleep SWA if different learning rates were present during wakeful afferent stimulation (Huber et al. 2004). Obviously, the amounts of wakefulness and afferent activity are important factors in determining the net synaptic changes, but they are not the only and not the main determining factors. In fact, the importance of the quality of wakefulness, the amount of new experiences, that subjects were faced during wakefulness, was shown to alter the subsequent slow-wave sleep, in a field study of Horne and Minard (1985). Subjects of the experiments of this study were unexpectedly involved in different playful activities: they had a car journey to another city, they visited a large exhibition center and a museum, and they were invited for a whole-day program in an amusement park and a zoo instead of boring paper-and-pencil tests. Although physical activity did not enhance during these programs, their effect on the subsequent slow-wave sleep was evident during the sleep laboratory examination (Horne and Minard 1985).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








