Circadian Rhythm Sleep Disorders
Kathryn J. Reid
Cathy Goldstein
Phyllis C. Zee
INTRODUCTION
Circadian rhythms in physiology and behavior are ubiquitous in all living organisms from single cells to humans. These self-sustaining endogenous circadian rhythms are genetically regulated and persist in the absence of external time cues, with a period slightly longer than 24 hours (1). In mammals, the suprachiasmatic nuclei (SCN), a paired structure located in the hypothalamus, is the site of a master circadian clock (2,3,4 and 5). The SCN not only generates circadian rhythms, but also maintains the temporal organization of circadian rhythms to the external physical, social, and work schedules. The nadir of the circadian core body temperature and the rise in melatonin rhythm are commonly used as estimates of the phase of the circadian clock in humans.
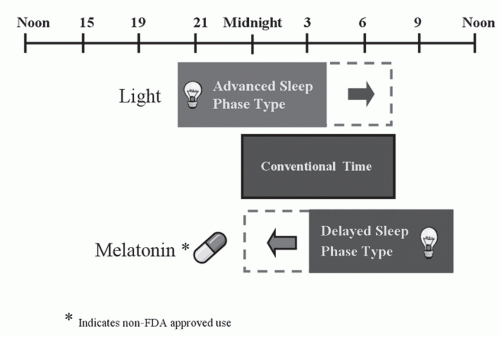
In humans, light is the strongest synchronizing agent for the circadian clock (6), and its ability to advance or delay circadian rhythms is dependent on the time of exposure. Exposure to light in the first half of the night delays circadian rhythms, whereas, light late in the second half of the night or early morning advances circadian rhythms. In humans, the transition point between the delay and advance regions occurs near the temperature minimum (4:00-6:00 AM) in young adults and somewhat earlier in older adults (7,8). The endogenous circadian clock is particularly sensitive to blue wavelength light, with light of 460 nm wavelength producing twice the shift in melatonin rhythms as longer wavelength light (9). In addition to light, nonphotic agents such as melatonin, and physical and social activity also play a role in entrainment of human circadian rhythms (10,11). The phase shifting responses to melatonin (12,13 and 14) are generally in the opposite direction of light. Early evening administration of melatonin will advance and early morning melatonin will delay circadian rhythms.
The sleep-wake cycle is the most apparent circadian rhythm in humans. During the past decade, there has been tremendous progress in our understanding of the neural regulation of sleep and wakefulness. Our current understanding of the regulation of the human sleep-wake cycle indicates that sleep and wake behaviors are generated by a complex interaction of endogenous circadian and sleep homeostatic processes, as well as social and environmental factors. The drive toward sleep and the tendency to sleep longer and more deeply after sleep loss is referred to as “sleep homeostasis” (process S). This homeostatic drive for sleep is a function of the amount of prior wakefulness. Physiological sleepiness and alertness not only varies with prior waking duration, but also exhibits circadian variation. In humans, daily variation in physiologic sleep tendency reveals a biphasic circadian rhythm of wake and sleep propensity (15,16), with a mid-day increase in sleep tendency and decrement in alertness occurring around 2 to 4 PM, followed by a robust decrease in sleep tendency and increase in alertness that lasts through the early to mid-evening hours. Finally, a nadir of the circadian alerting signal occurs 2 hours before waking. This circadian fluctuation (process C) interacts closely with process S, such that wakefulness is maintained in the evening when homesostatic drive for sleep is the most pronounced and sleep is sustained in the early morning hours despite dissipation of the homeostatic sleep need. Thus, the primary role of the circadian pacemaker is to promote wakefulness during the day and facilitate the consolidation of sleep during the nighttime hours (16,17,18 and 19). This two process model typically allows for approximately 16 hours of wakefulness and 8 hours of sleep each day.
CIRCADIAN RHYTHM SLEEP DISORDERS
For optimal sleep and alertness, desired sleep and wake times should be synchronized with the timing of the endogenous circadian rhythm. Misalignment between
the circadian timing system and the 24-hour physical environment or work and social schedules can result in symptoms of insomnia and excessive daytime sleepiness (EDS). Circadian rhythm sleep disorders (CRSDs) arise either when the physical environment is altered relative to the internal circadian timing system, such as in jet lag disorder and shift work disorder (SWD), or when the timing of endogenous circadian rhythms is misaligned with the external 24-hour day such as in delayed sleep phase disorder (DSPD), advanced sleep phase disorder (ASPD), free running disorder (FRD), and irregular sleep-wake disorder (Fig. 17-1). The latter is thought to occur predominantly because of chronic alterations in the circadian clock and its reactivity to entrainment mechanisms.
the circadian timing system and the 24-hour physical environment or work and social schedules can result in symptoms of insomnia and excessive daytime sleepiness (EDS). Circadian rhythm sleep disorders (CRSDs) arise either when the physical environment is altered relative to the internal circadian timing system, such as in jet lag disorder and shift work disorder (SWD), or when the timing of endogenous circadian rhythms is misaligned with the external 24-hour day such as in delayed sleep phase disorder (DSPD), advanced sleep phase disorder (ASPD), free running disorder (FRD), and irregular sleep-wake disorder (Fig. 17-1). The latter is thought to occur predominantly because of chronic alterations in the circadian clock and its reactivity to entrainment mechanisms.
The essential feature of a CRSD is that the pattern of sleep disturbance is due primarily to alterations of the circadian time-keeping system or a misalignment between the endogenous circadian rhythm and exogenous factors that affect the timing or duration of sleep. The circadian-related sleep disruption leads to insomnia or EDS, which causes functional impairment or distress. The diagnosis of CRSD is primarily based on published criteria using either the International Classification of Sleep Disorders, Second Edition (20) or the DSM IV-TR (21). In addition to physiologic and environmental factors, maladaptive behaviors often influence the presentation and clinical course of CRSDs.
Delayed Sleep Phase Disorder (Delayed Sleep Phase Type)
DSPD was first described in 1981 by Weitzman and colleagues (22,23). It is characterized by bedtimes and wake times that are usually delayed 3 to 6 hours relative to desired or socially acceptable sleep-wake times (Fig. 17-1). The patient typically cannot fall asleep before 2 to 6 AM and has difficulty waking up earlier than 10 AM to 1 PM (24,25). Attempts to advance sleep times are usually unsuccessful. When allowed to follow a preferred schedule, circadian phase of sleep is delayed, but relatively stable and sleep quality is reported to be normal. When the individual has no obligations, such as on weekends and vacations, sleep often is extended into the late morning. Patients with DSPD often report feeling most alert in the evening and most sleepy in the early morning. They typically score as definite “evening” types on the Horne and Ostberg questionnaire of diurnal preference and often are described as “night” people, or “owls” (26). There appears to be a high prevalence of depressive symptoms in patients with DSPD and an increased likelihood of seasonal affective disorder as compared to controls (27,28). In general, individuals with DSPD seek treatment because enforced socially acceptable bed times and wake up times result in insomnia, excessive sleepiness, and functional impairments, particularly during the morning hours (25).
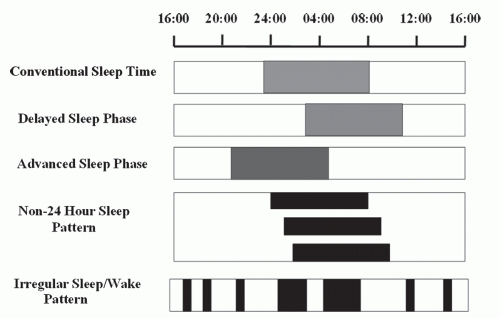 FIGURE 17-1 Schematic representation of the CRSD due primarily to alterations of the circadian timing system. |
Clinical Epidemiology
DSPD is probably the most common of the primary CRSDs (29). Although the actual prevalence of DSPD in the general population is unknown and may be as low as 0.17% (30), it has been reported that among adolescents and young adults, the prevalence is 7% to 16% (25,31). The greater prevalence in adolescence may be a consequence of both physiological and behavioral factors (32). Hormonal changes may be involved specifically, as delayed sleep phase is associated with the onset of puberty (when controlling for age) (33). It has been estimated that 5% to 10% of patients presenting with chronic insomnia to
sleep clinics have DSPD (24). DSPD may also be seen more frequently in populations with other neurological and medical disorders with delayed circadian rhythms observed in traumatic brain injury, Huntington’s disease, and patients with liver cirrhosis (33,34 and 35).
sleep clinics have DSPD (24). DSPD may also be seen more frequently in populations with other neurological and medical disorders with delayed circadian rhythms observed in traumatic brain injury, Huntington’s disease, and patients with liver cirrhosis (33,34 and 35).
Differential Diagnosis
DSPD must be distinguished from “normal” sleep patterns, particularly in adolescents and young adults, who exhibit delayed schedules without impaired functioning. Social and behavioral factors play an important role in the development and maintenance of the delayed sleep patterns. Behaviors, such as attempts to fall asleep earlier, result in prolonged sleep latency and may promote as well as perpetuate features of conditioned insomnia; activities and exposure to bright light into the late evening may promote the inability to sleep and exacerbate the delayed circadian phase. In adolescents, the role of school avoidance, social maladjustment, and family dysfunction must be considered as precipitating and contributing factors. Individuals may use alcohol and excessive caffeine to cope with symptoms of insomnia and excessive sleepiness, which, in turn, may exacerbate the underlying CRSD.
Etiology
Although the exact mechanisms responsible for DSPD are unknown, several explanations, such as a longer than usual endogenous circadian period or alterations in the entrainment of the circadian system, could account for a persistently delayed-phase relationship between the endogenous circadian rhythm and the desired or conventional times for sleep and wake (25). It has been suggested that the advance portion of the phase response curve to light may be unusually small (24) or lack of early morning light exposure (due to prolonged sleep in the morning) may promote the delayed-sleep phase under normal light-dark cycles. Furthermore, individuals with DSPD may have an altered responsiveness to light with hypersensitivity to evening light and an impaired response to morning light (36). Therefore, there is evidence that changes in oscillation of the circadian clock and/or the response to synchronizing agents, such as light, contribute to the alteration in the timing of sleep in DSPD.
Although circadian mechanisms are fundamental in the pathophysiology of DSPD, there is increasing evidence that alterations in the homeostatic regulation of sleep may also play an important role (37). It is commonly accepted that sleep architecture is essentially normal in patients with DSPD. Polysomnographic (PSG) recordings of sleep in DSPD patients showed that sleep architecture was not disrupted when subjects were allowed to sleep at their desired sleep and wake times (23,38,39 and 40). Of note, following sleep deprivation, DSPD patients showed a decreased ability to compensate for sleep loss during the day and first hours of the night (41,42). In addition, increased slow wave sleep in the latter part of the night in patients with DSPD is also suggestive of impairment in homeostatic regulation of sleep (43). However, it has been difficult to reproduce these findings consistently (44). Futhermore, a difference in the magnitude of delay in phase markers despite similarly delayed sleep-wake times has been noted in the literature, possibly hinting at heterogeneity in the pathophysiology of patients with DSPD (45).
A family history may be present in approximately 40% of individuals with DSPD, and the DSPD phenotype has been shown to segregate as an autosomal dominant trait (46). Further evidence of a genetic basis for DSPD comes from recent reports of polymorphisms in the circadian clock genes, hPer3 and Clock in DSPD (47,48,49 and 50). Polymorphisms, in not only the coding region, but also (as recently described) the promoter region of the human PER3 gene, associate with the DSPD phenotype (51). In addition, a single nucleotide polymorphism in the arylalkylamine N-acetyl transferase gene was found more frequently in patients with DSPD as compared to controls (48). HLA-DR1 positivity has also been associated with DSPD in certain groups (52,53).
Diagnostic Evaluation
The diagnosis of DSPD relies largely on the clinical history. However, diagnostic studies such as sleep diaries and actigraphy can be very useful to confirm the delayed sleep-phase pattern. Recordings of sleep diaries and actigraphy over a period of at least 2 weeks demonstrate delayed-sleep onset and offset (Fig. 17-1), with sleep onsets typically delayed until 2 to 6 AM and wake up times in the late morning or early afternoon. Daily work or school schedules may result in earlier than desired wake time during weekdays, but a delay in bedtime and wake up time is almost always seen during weekends and while on vacation. In 2007, the American Academy of Sleep Medicine (AASM) published practice parameters regarding the use of actigraphy, suggesting its use as a diagnostic tool in DSPT and as an assessment measurement for treatment outcomes in CRSDs as a guideline (54). Overnight polysomnogram is not routinely indicated in the evaluation of DSPD; however, in the setting of concurrent sleep maintenance insomnia and daytime somnolence it is essential to rule out other sleep disorders such as sleep apnea and periodic limb movement disorder. When performed at the preferred delayed sleep times, PSG parameters of sleep architecture are usually normal for age (23,38,39 and 40). However, if a conventional bedtime and wake up time is scheduled, PSG recording will show prolonged sleep latency and decreased total sleep time (TST).
Measurements of circadian phase can be performed by assessment of core body temperature or dim-light melatonin onset (DLMO) in plasma or saliva. These circadian measures typically show the expected phase delay in the timing of these circadian rhythms. In addition to physiological measures, the Horne-Ostberg questionnaire is a useful tool to assess the “circadian type” of “eveningness” and “morningness” (26,55). Individuals with DSPD score as definite evening types.
Clinical Management
Approaches aimed at resetting circadian rhythms, such as chronotherapy, timed bright light, and melatonin have been employed for the treatment of DSPD. Chronotherapy is a treatment in which sleep times are progressively delayed by approximately 3 h/d until the desired earlier bedtime schedule is achieved (23). Although effective, the length and repeated nature of treatment and need for adherence to restrictive social and professional schedules limit practicality in the clinical setting. However, in adolescents, in which behavioral factors often contribute to the delayed-sleep phase, chronotherapy in conjunction with enforcement of regular sleep and wake times are important components of the clinical management. The AASM suggests chronotherapy as an option for treatment of DSPD (56).
Exposure to bright light for 1 to 2 hours in the morning results in an advance of the phase of circadian rhythms, whereas evening light exposure causes phase delays. Therefore, bright light exposure during the early morning hours and avoidance of bright light in the evening have been shown to be effective treatments for DSPD (57,58) (Fig. 17-2). Following 2 weeks of exposure to 2 hours of bright light of 2,500 lux each morning and restricted evening light, individuals with DSPD showed earlier sleep times and reported improved morning alertness level (58). However, many patients, particularly those who are severely delayed, find it difficult to awaken earlier for the 1 to 2 hours of bright-light therapy. Despite the potential utility of bright-light therapy, the timing, intensity, and duration of treatment remain to be defined. Exposure to broad-spectrum light of 2,000 to 10,000 lux for approximately 1 to 2 hours is generally recommended for use in clinical practice. The AASM suggests morning light exposure as a guideline for the treatment of delayed sleep phase syndrome (56).
Due to the practical limitations of chronotherapy and phototherapy, melatonin, taken orally in the evening, has been increasingly investigated as a treatment for DSPD. Several studies have demonstrated the potential benefits of melatonin administered in the evening (59,60,61,62 and 63). In particular, in a double-blind study, circadian phase markers were significantly advanced with both 0.3 and 3 mg of melatonin with greatest advances occurring at earlier adminstration times (6.5 hours) in relation to DLMO (61). In a recent meta analysis of placebo controlled studies using melatonin for DSPD, a mean advancement of 1.69 hours was seen (64). Use of melatonin timed appropriately is recommended as a guideline for treatment of DSPD (56). In addition, in individuals with comorbid depressive symptoms, melatonin may improve mood as well as sleep (65). There is currently inadequate evidence to suggest the use of hypnotics to facilitate sleep onset and stimulants to promote wakefulness in DSPD (66).
Treatment success in DSPD depends on many variables, including severity of the delayed-sleep phase, comorbid psychopathology, ability and willingness of the patient to comply with the treatment, school schedule, work obligations, and social pressures (25,67 and 68). A summary of treatment approaches of DSPD is shown in Figure 17-2.
Treatment success in DSPD depends on many variables, including severity of the delayed-sleep phase, comorbid psychopathology, ability and willingness of the patient to comply with the treatment, school schedule, work obligations, and social pressures (25,67 and 68). A summary of treatment approaches of DSPD is shown in Figure 17-2.
Advanced Sleep-Phase Disorder (Advanced Sleep Phase Type)
ASPD is a sleep disorder in which there is a stable advance of the major sleep period, characterized by habitual and involuntary sleep onset and wake-up times that are several hours earlier relative to conventional and desired times (Fig. 17-1). Individuals with ASPD usually report sleep onset around 6 to 9 PM and wake time of 2 to 5 AM (69 and 70). ASPD complaints include early-morning awakenings, sleep maintenance insomnia, and also of sleepiness in the late afternoon or early evening. Individuals with ASPD typically consider themselves “larks” and score as morning types on the Horne-Ostberg questionnaire (26).
Clinical Epidemiology
The actual prevalence of ASPD is estimated at much lower than DSPD (30,71). ASPD not associated with aging is probably rare, with only a few reported cases (70,72,73). This condition is more common among middle-age and older adults, with an estimated prevalence of 1% of middle-age adults (71). However, it is not known whether the more commonly seen age-associated advance in sleep and wake times is the same entity as ASPD in younger individuals. Recently, in one study, African Americans were found to have a shorter circadian period and larger magnitude phase advances to light than Caucasians. Therefore, it is possible that the propensity to develop ASPD could differ based on race (74).
Differential Diagnosis
ASPD should be distinguished from “normal” sleep patterns, particularly in the elderly, who maintain advanced schedules without distress or impaired functioning (morning types or larks). Because depression may also present with early morning awakening, it is important to distinguish ASPD from depression and other types of mood disorders.
Etiology
Although the precise mechanisms underlying the pathophysiology of ASPD are unknown, several circadian-based mechanisms, such as an unusually short endogenous circadian period that is <24 hours (72), and decreased exposure or weakened response to entraining agents, such as light and physical activity (75,76 and 77), may impair phase delays and promote an advanced sleep phase. In addition to an earlier circadian phase, there is evidence that homeostatic regulation of sleep is also altered in older adults with advanced sleep phase (78).
Diagnostic Evaluation
In addition to the history, sleep diaries and actigraphy can be very useful to confirm the advanced sleep-phase pattern. Recordings of sleep diaries and actigraphy over a period of at least 2 weeks demonstrate advanced sleep onset and offset (Fig. 17-1), with typical sleep onsets of 6 to 9 PM and wake times of 2 to 5 AM. Actigraphy is suggested as a guideline by the AASM for its use in the evaluation of ASPD and as an assessment for treatment response (54).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree