Fig. 1
The skin incision covers the suboccipital region and the arch of C1
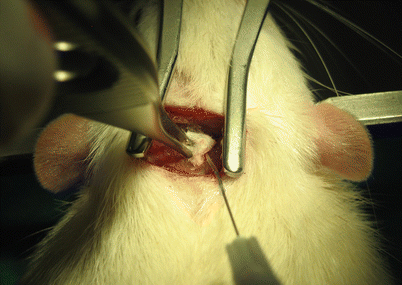
Fig. 2
Induction of subarachnoid hemorrhage by the injection of autologous arterial blood into the cisterna magna
An identical surgical procedure is repeated 24 h after the initial SAH for induction of the second SAH. During the subsequent observation or treatment course, animals receive 5 ml crystalloid solution and 0.0125 mg fentanyl subcutaneously twice a day.
Technical Considerations
An initial small skin incision ensures less surgical trauma and, therefore, a reduction of stress to the animals. The use of a flexible polythene tube for injection of autologous blood into the cisterna magna might minimize the risk of brainstem injuries. The leakage of injected arterial blood can be avoided by adding fibrillar haemostypticum patches (Tabotamp®) and cotton. The injected blood volume should not exceed 0.25 ml to avoid collateral damage.
Technical Modifications
Several technical modifications have been reported for the rat double-hemorrhage model. For instance, unilateral common carotid artery occlusion (CCAO) is known to cause moderate reduction of CBF in both cerebral hemispheres without asymmetrical perfusion [16]. A recent study demonstrated that CCAO deteriorates the effects of CVS in the rat double-hemorrhage model and therefore leads to an aggravation of CVS-related delayed brain tissue damage [17].
A modified rat double-hemorrhage model using a catheter-based blood injection into the cisterna magna through a parieto-occipital burr hole was suggested recently. This modification showed presumably lower mortality rate despite technical difficulties with the positioning of the catheter [18]. To reduce the confounding effects of surgical procedures, a modification using minimally invasive blood injection by a percutaneous, stereotactic injection technique has been reported [19].
Outcome Measures
Neurological Assessment
Delayed consequences of SAH are clinically diagnosed by the so-called delayed ischemic neurological deficit (DIND). Outcome measures include neurological scores, usually investigating motor deficits. For the rat double-hemorrhage model, scales by Ryba and Bederson were described and regularly used [20, 21]. Additionally, the rotating pole test allows prediction of functional motor deficits [22, 23]. Furthermore, Garcia reported a more detailed score for assessment of neurological deterioration in rats with middle cerebral artery occlusion [24]. Thereby, rats were scored from 0 to 3 in six neurobehavioral tests investigating spontaneous activity, symmetry in the movement of all four limbs, forepaw outstretching, climbing, body proprioception, and response to vibrissae touch.
Radiological Investigations
The gold standard in radiological investigations to detect CVS is digital subtraction angiography (DSA). The challenge in angiographic investigations of rat cerebral arteries because of the small vessel diameter has been discussed previously and several techniques have been investigated [15, 25, 26]. Selective catheterization of the vertebral artery (VA) shows the highest resolution in imaging the basilar artery (BA). However, multiple angiographic investigations contain a higher risk of cardiac abnormalities caused by the contrast medium. Therefore, the use of one selective DSA at the end of the experiment seems to represent a practical reference parameter for the quantitative determination of delayed CVS in the rat. The time course of the development of angiographic CVS in this model has already been characterized, and the maximum CVS was found to occur on day 5, in agreement with morphological investigations using immunohistochemistry [7, 8].
However, cerebral perfusion measurement by computed tomography (CT) or magnetic resonance (MR) imaging are becoming more important for diagnosis and monitoring of impaired CBF after SAH during the course of clinical treatment of patients with SAH. Accordingly, these tools have also been applied in the animal model [7, 27, 28].
Furthermore, determination of CBF and/or cerebral blood volume (CBV) is germane to detect delayed pathophysiological effects of CVS. The application of a noninvasive MR perfusion-weighted imaging (PWI) method was the first to characterize the decrease in CBF and CBV in the rat double-hemorrhage model in a semiquantitative fashion. Both CBV and CBF are reduced to approximately one-third of the control value on day 5 after the initial bleeding [7].
Histological Investigations
Histopathological investigations are used to investigate the morphological changes after SAH. Because of their known sensitivity to cerebral ischemia, vital neurons were counted in the hippocampus and adjoining cortex regions for the assessment of CVS-related cerebral ischemia [29–31]. Neurons were usually classified as nonvital or necrotic in the presence of pyknosis, karyorrhexis, karyolysis, cytoplasmic eosinophilia, or loss of affinity for hematoxylin [32]. In the rat double-hemorrhage model, a significantly decreased neuronal cell count was observed in the hippocampal regions and inner cortex layers as result of delayed cerebral damage caused by CVS on day 5 but not on day 3 [8].
Apoptosis as a result of delayed brain injury caused by CVS was detectable using the TUNEL-staining method in a histopathological investigation approximately 7 days after SAH [33]. Furthermore, the inner vessel diameter and wall thickness of the BA are commonly used as histological parameters to indicate CVS. Consistent with angiographic results, the reduction of the arterial diameter in histological investigations is approximately 50 % [7, 8, 34].
Advantages and Limitations
This rat double-hemorrhage model has several limitations that have previously been discussed [14].
First, double-injection models seem less suitable to investigate the acute stage of SAH, including EBI [14]. However, Prunell et al. demonstrated a great variation in the amount of subarachnoid blood in the different injection and perforation models and a faster restoration of CBF after SAH induction in the injection models compared with the perforation models [37]. Although reflecting the clinical setting, great variation of subarachnoid blood volume might lead to the need for larger groups. The mortality rate in this rat double-hemorrhage model has been described to be up to 50 % in previous studies [7, 8, 11]. This mortality rate is even increased by the use of invasive procedures, such as DSA. The high mortality rate might have been criticized, but it also indicates the implementation of severe experimental SAH and is comparable to the outcome in humans.
Because of the known pronounced cortical collateralization in the rat, ischemic territorial infarctions do not occur [20]. Nevertheless, because of several advantages, the rat double-hemorrhage model is a feasible, effective, and customizable rodent model for experimental SAH. First, the experimental setting is cost effective, manageable, and can be established in most centers. Furthermore, this model provides a good imitation of the clinical setting and time course of delayed effects of CVS. Eventually, the degree of severity and characteristics of CVS as well as the reduction of CBF are more pronounced in the rat double-hemorrhage model when compared with single-injection models [5].
Conclusion
The aim of rat subarachnoid blood injection models is mainly to imitate CVS or delayed cerebral perfusion deficits after SAH. The rat double-hemorrhage model elongates the time course of the pathophysiological consequences of CVS, which imitates the clinical setting more precisely than single-hemorrhage models. The possibilities of extended investigations in this model, e.g., by in vivo evaluations such as MRI or DSA, considerably increases the complexity of the model but imitates the clinical course in humans more closely than other established rodent models. Therefore, the double-hemorrhage model is used to mimic the delayed effects of SAH and to investigate the use of drugs on morphological ischemic, functional, and vasospastic effects.
Conflict of Interest Statement
We declare that we have no conflict of interest.
References
1.
2.
Macdonald RL, Kassell NF, Mayer S, Ruefenacht D, Schmiedek P, Weidauer S, Frey A, Roux S, Pasqualin A, Investigators C– (2008) Clazosentan to overcome neurological ischemia and infarction occurring after subarachnoid hemorrhage (CONSCIOUS-1): randomized, double-blind, placebo-controlled phase 2 dose-finding trial. Stroke 39(11):3015–3021PubMedCrossRef
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





