Fig. 4.1
Hand motor cortex mapping before and after a series of contralesional M1 LF 1 Hz rTMS. S1 denotes primary somatosensory cortex. In this case of a 75-year-old female patient, 5.5 weeks post right hemisphere subcortical stroke, mapping of the hand motor areas of the stroke hemisphere was performed before (pre) and after (post) a series of 1 Hz rTMS (110 % RMT, 900 stimuli, neuronavigated at the M1 hot spot of the abductor pollicis brevis muscle, APB in the non-lesioned hemisphere) for 18 sessions in 4 weeks. The orange target denotes the APB ‘hot spot’ based on the examination at ‘post’. Mapping has been performed with 110 % of the RMT. Grey target denotes stimulation points with no MEPs (<50 μV); red targets indicate stimulation points where MEPs with an amplitude between 50 and 500 μV could be elicited (MEPs with amplitudes >500 μV could not be elicited in this case). Note that prior to rTMS no MEP could be evoked while there was an APB map with 15 active points (spaced 0.5 cm apart) after the series of contralesional M1 LF 1Hz rTMS. Clinically, the patient did not have active hand and only poor arm motor control prior to the rTMS series (Fugl-Meyer arm motor score, FM: 9; Action Research Arm test, ARAT: 0), while there was some active hand motor control afterwards (FM: 14; ARAT: 3). rTMS and mapping were performed with the Nexstim therapeutic system (Nexstim TM, Finland)
4.2.1.2 High-Frequency rTMS of the Ipsilesional Motor Cortex (HF-rTMS)
Kim and colleagues (2006) showed as a ‘proof of principle’ in a cross-over laboratory experiment with single sessions of ipsilesional M1 sham or 10 Hz rTMS (160 pulses, 2 s trains at 10 Hz with 68 s inter-train interval, 80 % RMT FDI) paired with 40 s practising a finger sequence task (during inter-train intervals) that 10 Hz rTMS can enhance excitability and short-term motor plasticity in mildly affected (chronic) stroke patients.
The effects of repeated HF-rTMS of the ipsilesional motor cortex in subacute stroke (<1 month) with mild to severe arm paresis on both arm motor recovery, as well as leg motor recovery, mobility and independence with activities of daily living, were investigated in a single blind RCT with 28 patients by Chang and colleagues (2010). A daily dose of 1,000 pulses of subthreshold ipsilesional 10 Hz rTMS combined with training (90 % RMT FDI, 5 s trains at 10 Hz with 55 s inter-train intervals consisting of 50 s reaching and grasping exercises and 5 s rest) was applied for 10 days within 1 month after onset of stroke, at the FDI hot spot or a corresponding mirror position of the non-lesioned hemisphere. Pre, post and 3 months follow-up assessments included motor clinical scales (Motricity Index arm and leg, Fugl-Meyer motor score, arm and leg, the box and block test [BBT], the functional ambulation category [FAC]) and an ADL scale (Barthel index, BI). A differential beneficial effect of real vs. sham HF-rTMS was documented for the Motricity Index, arm score only. Adverse effects were not observed. The findings indicate that subthreshold HF-rTMS of the ipsilesional arm motor cortex in subacute stroke patients can be safe and seems to enhance specifically (long-term) recovery of mild to severe arm paresis.
Sasaki and coworkers also included acute/subacute stroke patients comparing the effects of 5 days ipsilesional M1 10 Hz rTMS (1,000 pulses, 10 s trains at 10 Hz with 50 s inter-train interval, 90 % RMT), contralesional M1 1 Hz rTMS (1,800 pulses, 90 % RMT) and sham stimulation on finger tapping and grip strength. Again, adverse effects were not observed. For these subcortical ischaemic or haemorrhagic stroke patients, both types of real rTMS groups led to an increase in grip strength and finger tapping speed. Only for the 10 Hz rTMS group were changes in grip strength and tapping significantly different from the sham group, hinting to a better substantiated effect.
In summary, the data on HF-rTMS from RCTs is still limited, nevertheless indicating some clinical benefit. A clinical safety concern that might have prevented a more frequent use of HF-rTMS in clinical trials in motor stroke is its theoretically higher potential to induce epileptic fits (excitatory stimulation applied to the affected hemisphere) when compared to LF-rTMS to the contralesional M1 (inhibitory stimulation applied to the non-affected hemisphere). HF-rTMS of the ipsilesional M1 (1,000 pulses, 10 Hz, 80–90 % RMT FDI) was, however, not associated with any severe adverse event in the reported trials. It seemed to improve strength (grip strength, MI arm) and speeded selective movement (tapping) specifically and might induce long-term effects.
4.2.1.3 Theta-Burst Stimulation (TBS)
Talleli and colleagues (2007) compared single contralesional M1 cTBS and ipsilesional M1 iTBS with sham treatments (without motor training) on cortical excitability and motor performance measures in a small sample of 6 chronic stroke patients with mild arm paresis. Only ipsilesional iTBS improved motor behaviour (shorter simple reaction time, SRT) of the paretic hand and changed physiological measures, i.e. increased excitability on stroke side, compared to sham stimulation. Grip strength and complex reaction time (CRT) were not differentially changed. cTBS reduced transiently motor-evoked potentials (MEP) of the healthy hand. The small sample size, number of stimuli (cTBS-300) and lack of training combined with stimulation are limitations of this laboratory experiment. The data suggests effects of both iTBS and cTBS without a clear therapeutic indication.
Among ten mild to moderately hemiparetic patients with chronic subcortical stroke, a single cTBS (reverse coil orientation) of the contralesional, a single iTBS (conventional orientation) of the ipsilesional M1 (600 stimuli, 90 % active motor threshold [AMT] FDI), or a sham stimulation was followed by 4 × 4 min practising precision grip movements (Ackerley et al. 2010). iTBS increased MEP amplitudes while the arm activity score ARAT was unchanged. After cTBS MEPs amplitudes as well as the ARAT score were on average reduced. While rather an experimental than a therapeutic setting (single TBS session), the example shows that TBS can affect MEPs and arm activity, that coil orientation and interaction with activity matter and that TBS can at times have a detrimental effect on function.
Given the network structure of sensorimotor control, it is conceivable that stimulation of different ‘nodes’ in sensorimotor networks could have differential effects on motor learning (Platz et al. 2012a, b) and motor recovery. Meehan and coworkers (2011) asked 12 chronic stroke patients with mild to moderate arm paresis to practise a serial target task (STT) for 3 days while receiving a sham or cTBS stimulation to either the contralesional M1 or S1 a couple of minutes before starting to practise. Both real cTBS groups showed bigger improvements with both the practised STT and with regard to completion time of the Wolf motor function test (WMFT) as compared to sham. Interestingly, the kinematics of the movements (movement time, maximal velocity, acceleration and deceleration) showed a bigger practice effects after contralesional M1 cTBS as compared to contralesional S1 cTBS, while movement initiation time and time to complete the WMFT tasks showed bigger improvements after cTBS to S1. Accordingly, different aspects of sensorimotor control of stroke patients might differentially be influenced by neuronavigated cTBS of either the contralesional S1 or M1.
Talelli and colleagues (2012) conducted a randomised sham-controlled trial involving 41 chronic stroke patients with mild to moderate hand motor deficits and blinded assessment. For 10 daily sessions all patients received strength training for wrist, fingers and thumb of the paretic hand as well as repetitive grasp and task practice including reaching. The training was primed by either sham stimulation, ipsilesional iTBS or contralesional cTBS at the FDI hot spot. Overall small but sustainable improvements were corroborated and shown to outlast the training period at least until 30 days later (NHPT, JHFT, grip strength [not pinch grip]; goal attainment scale [GAS] and VAS (patient satisfaction) assessed after treatment only). The training effects were, however, small and below the preset level of clinical significance (set at 10 % of each test’s maximum). No effects of either iTBS or cTBS as compared to iSham or cSham could be corroborated. Thus, in this clinical situation of chronic stroke patients with mild hand and arm paresis who received a specific training for 10 days, iTBS or cTBS priming was not beneficial. TBS seems therefore not to have induced an (purely) additional effect on motor improvement; any potential modifying effect might not have become observable giving the small effects of training itself.
Sung and coworkers investigated the effects of a combined first inhibitory than excitatory treatment course in stroke patients in the late subacute/early chronic phase (<1 year). Randomly assigned four groups participated in 20 daily sessions (4 weeks), receiving during the first 10 days (1st course) either real contralesional M11Hz rTMS or sham, followed by another 10 days (2nd course) with either real ipsilesional M1 iTBS or sham. The groups receiving either or both rTMS courses had bigger improvements on various motor outcome measures (WMFT, FM arm, finger tapping and reaction time) than the group receiving sham only. The group receiving both 1 Hz rTMS and then iTBS had bigger improvements (WMFT and RT) than the groups receiving one rTMS course (1Hz rTMS or iTBS). Motor map area decreased contralesionally after 1 Hz rTMS and was enlarged ipsilesionally after iTBS. Results were not modified by the factors cortical versus subcortical or ischaemic versus haemorrhagic stroke. It can be concluded that either 2 weeks of contralesional M1 1 Hz rTMS or ipsilesional M1 iTBS produced motor map area changes and motor improvements in these subacute to early chronic ischaemic or haemorrhagic stroke patients, with the prolonged/combined treatment producing substantially bigger behavioural effects.
In a consecutive double-blind RCT with 48 subacute ischaemic stroke patients with moderate to severe arm paresis (MRC ≤3), Wang and colleagues (2014) observed that both a sequence of 2 weeks contralesional M1 1 Hz rTMS followed by 2 weeks ipsilesional M1 iTBS as well as the reverse sequence produced motor map area changes and substantial and sustainable motor improvements (MRC, FM arm, WMFT) compared to sham. Motor recovery was, however, considerably bigger after the sequence of first 10 daily session contralesional M1 1 Hz rTMS followed by ipsilesional M1 iTBS (appr. 50 % improvement after the intervention period and 60–70 % at 3 months post) compared to the reverse order (20–30 % improvement). The sham group showed only small improvements (<10 % on average) indicating that the applied physiotherapy itself was not very effective.
Taken together, neither the inhibitory protocol cTBS when applied to the contralesional M1 nor the excitatory iTBS when applied to the ipsilesional M1 had effects on motor control and recovery been consistent across trials. Further, any specific effect on sensorimotor control in stroke patients with arm paresis could be modified by the stimulation target, e.g. contralesional M1 or S1 for cTBS. Most interesting clinically are the two RCTs from Taiwan (Sung et al. 2013; Wang et al. 2014) where a substantial number of stroke patients received combined rTMS and PT sessions over a total of 4 weeks. The prolonged combination of rTMS with 10 daily sessions of contralesional 1 Hz rTMS, followed by 10 daily sessions of ipsilesional M1 iTBS, led to the best observed, substantial and long-term motor recovery (50–70 % improvement compared to <10 % in the sham only control group). These results suggest that a prolonged priming of arm training both with a course of contralesional inhibitory and then ipsilesional excitatory rTMS might enhance motor recovery in subacute stroke patients.
4.2.1.4 Recovery of Gait
Chieffo and colleagues (2014) assessed the safety and efficacy of bilateral, excitatory, high-frequency rTMS over the lower limb cortical motor representation in 10 persons with chronic (>6 months) subcortical MCA stroke who were able to walk independently short distances (with aids if necessary). Each subject received both real and sham rTMS in a random sequence. The 2 rTMS cycles (real or sham) were composed of 11 sessions each, administered over 3 weeks and separated by a 4-week washout period. To reach the lower limb cortical motor areas, deeply located in the mesial cortical surface of the hemispheres, they delivered rTMS using a ‘Hesed coil’ (H-coil), which is designed to effectively stimulate at about a depth of 3–5 cm below the skull. HF-rTMS (30 trains at 20 Hz, 60 s inter-train interval, 1,500 pulses, 90 % RMT of either TA or 82 % max. stimulator output) was not specifically paired with motor exercises. Prior and after each treatment period and at a 4-week follow-up the Fugl-Meyer, leg motor score was assessed along with a 10 m walk test (10MWT) assessing gait velocity and a 6-min walk test (6MWT) measuring endurance. No adverse effects were observed. Superiority of improvement in favour of the real rTMS both after the treatment period and at follow-up 4 weeks later was documented for the Fugl-Meyer, leg motor score (only). The data suggest a potential of high-frequency rTMS delivered with the H-coil to both leg motor cortices for improving lower limb motor function in chronic ambulatory MCA stroke patients.
4.2.2 Meta-analyses
The systematic review and meta-analysis by Adeyemo and colleagues (2012) focused on treatment effects of rTMS (no TBS included) and tDCS on motor function after stroke and included studies published within 10 years, written in English, and involving at least three patients. Fifty studies with a total of 1,282 stroke subjects and an average age of 58.46 years were included. Only six studies included subacute patients, five acute patients. Thus, the evidence was largely covering chronic stroke patients.
No major adverse effects have been reported. The side effects reported were tingling, headache, dizziness, itching and increase in anxiety.
Most of the studies used small sample sizes. Thirty-six (72 %) studies used rTMS (the others tDCS). Most of the rTMS studies were controlled and used sham stimulation or active control stimulation (77.7 %); the techniques used were different: active coil placed on the vertex; active coil, with an angle of application of 90°; and sham coil, which induces no magnetic field.
A majority of the results was positive with bigger improvements after active rTMS compared to the control stimulation, with the exception of three articles (Lomarev et al. 2007; Malcolm et al. 2007; Pomeroy et al. 2007). The results from a fixed effects model revealed a significant pooled effect size of 0.584 (95 % CI, 0.440, 0.729) in favour of rTMS/tDCS. The random effects model showed similar results 0.590 (pooled effect size, 95 % CI, 0.421, 0.760). The authors found no evidence of publication bias.
The effect size was not influenced by age. Similarly, no robust effect of gender was reported with a slight hint towards bigger effects with a higher male proportion in study samples. Given the low number of studies investigating acute or subacute stroke patients, chronicity as a potential modifying factor could not rigorously be analysed. The (positive) evidence regarding long-term effects had been limited.
The analysis did, however, demonstrate a significantly increased effect size when stimulation was applied to subcortical strokes versus the mixed strokes. It is conceivable that a subcortical stroke that preserves the cortex allows rTMS to influence the recovery of functionally relevant cortical network activity and connectivity.
In conclusion, this review provides a broad picture including all sorts of rTMS approaches in motor stroke, and it gives an indication that there is a potential for a clinical benefit with an overall moderate effect size. The type of studies included (e.g. some laboratory, some clinical trials, not all randomised, limited blinded assessment, various stimulation types, limited information on long-term effects) all make it difficult to draw firm conclusions on whom to treat when, how and for how long and how to combine rTMS with training.
The systematic review and meta-analysis by Hao and colleagues (2013) assess the efficacy and safety of rTMS for improving function in people with stroke. The authors included only RCTs, trials comparing rTMS therapy with sham therapy or no therapy, and excluded trials that reported only laboratory parameters. The included studies could target motor function with rTMS as well as visual perception (neglect), aphasia or depression, all reflecting some type of ‘function’. Primary outcomes were activities of daily living (ADL), such as the Barthel index, the Functional Independence Measure and the modified Rankin Scale. Secondary outcomes were upper and lower limb motor function, any other improvement of impairment, adverse events, death or disability. Compared to the systematic review of Adeyemo and colleagues (2012), this review was methodologically more focused (only rTMS, only RCTs), but less focused regarding the target symptoms: The outcome measures were primarily addressing effects on ADLs, and only as secondary measures motor and cognitive function, or mood. Further, brain targets for rTMS were not restricted to M1. Hao and colleges included 19 trials involving a total of 588 participants in their review.
The quality of reporting in the trials in general was considered poor. The funnel plots showed a slightly asymmetrical funnel distribution, which indicated likely publication bias.
Eight trials with a total of 173 participants reported motor function of the affected extremities. However, data for a meta-analysis were available from only four trials and 73 participants (42.2 %, 73/173) (Fregni et al. 2006; Khedr et al. 2009; Malcolm et al. 2007; Pomeroy et al. 2007). This meta-analysis showed that rTMS treatment was not associated with a significant improvement in motor function (SMD 0.51, 95 % CI −0.99 to 2.01). However, there was statistically significant heterogeneity between trials (I 2 = 87.6 %).
Eight trials reported that there were no adverse effects. Six trials reported adverse outcomes: eight transient or mild headaches (2.4 %, 8/327) were observed in the rTMS group; one participant reported an increase in anxiety (0.3 %, 1/327); two participants had single episodes of neurocardiogenic syncope (0.6 %, 2/327) with their initial exposure to rTMS; an exacerbation of initial insomnia was observed in one participant (0.3 %, 1/327); and local discomfort at the site of the stimulation. Five trials made no mention of adverse outcomes.
In summary, this systematic review and its meta-analyses highlights the methodological quality restrictions in some of the rTMS trials and asks for methodologically more rigorous research in the field. It does, however, not focus on motor recovery after stroke and therefore its applicability in this domain is limited.
The systematic review and meta-analysis by Hsu and co-authors (2012) investigated (more) specifically the effects of repetitive transcranial magnetic stimulation (rTMS) on upper limb motor function in patients with stroke. They included only RCTs, studies needed to have a focus on upper limb function after stroke (had to recruit at least six patients and to be written in English).
Eighteen studies were identified. In total, 392 patients with stroke were included, and 370 were re-evaluated postintervention. Three studies recruited patients in the acute phase, three studies in the subacute phase and seven other studies investigated patients with chronic stroke. Regarding lesion sites, six trials recruited patients with subcortical stroke only, whereas the other studies recruited patients with both cortical stroke and/or subcortical stroke.
Thirteen of the 18 studies reported adverse effects. Only one trial found adverse events, including two patients with headaches, one patient with increased anxiety and one patient with increased fatigue.
The meta-analysis of motor outcome showed a statistically significant mean effect size of 0.55 (95 % CI, 0.37–0.72; P < 0.01).
Sub-analyses revealed the following results (compare Fig. 4.2): The analysis revealed a mean effect size of 0.69 (95 % CI, 0.42–0.95; P < 0.001) for patients who received low-frequency rTMS; the mean effect size for patients who received high-frequency rTMS was 0.41 (95 % CI, 0.14–0.68; P < 0.01). The subgroup mean effect size for acute stroke was 0.79 (95 % CI, 0.42–1.16; P < 0.001), 0.63 (95 % CI, 0.18–1.08; P < 0.01) for subacute stroke and 0.66 (95 % CI, 0.31–1.00; P < 0.001) for chronic stroke. The mean effect size for subcortical lesions was 0.73 (95 % CI, 0.44–1.02; P < 0.001), for nonspecified lesion sites 0.45 (95 % CI, 0.23–0.67; P < 0.001).
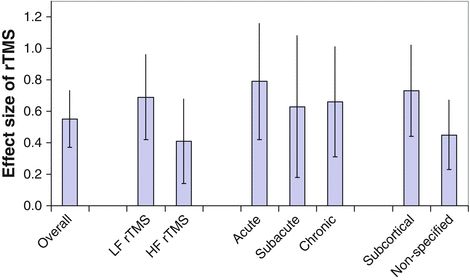

Fig. 4.2
Effect sizes for rTMS in arm motor rehabilitation after stroke according to a meta-analysis by Hsu et al. (2012). Standardised effect sizes and 95 % confidence intervals based on data from 18 trials and 370 patients are presented. Because effect sizes may be influenced by sample sizes and effects may be overestimated in studies with low numbers of patients, a weighting factor was applied that gave more weight to studies with larger samples. Finally, the mean effect sizes were obtained after combining the weighted effect size of each study. Absolute effect sizes that ranged from 0.2 to 0.49 were considered to be small, and a value of 0.5 was likely to be clinically meaningful (Sloan et al. 2005)
The effect of rTMS on cortical excitability was evaluated based on resting motor threshold data (RMT) from the affected hemisphere in six trials. The meta-analysis for RMT showed a non-significant mean effect size of 0.30 (95 % CI, −0.09 to 0.68; P > 0.05).
For all mentioned analyses, there was no heterogeneity across the studies.
Although the above-mentioned subgroup analysis indicated a greater beneficial effect of contralesional low-frequency rTMS compared with ipsilesional high-frequency rTMS, the TBS studies revealed that ipsilesional iTBS may be more helpful for motor recovery (no formal analysis performed due to limited data).
From this focused meta-analysis including RCTs that specifically assessed the effects of rTMS (including TBS) on upper limb motor function after stroke, it can be concluded that the intervention tested has a moderate positive effect (mean effect size 0.55). There are factors that are associated with somewhat higher effect sizes (subcortical stroke, acute stroke, contralesional LF-rTMS) and yet a positive effect of rTMS could still be corroborated in subgroups without these ‘positive’ factors, i.e. with stroke involving the cortex, chronic stroke, ipsilesional HF-rTMS. Long-term effects are, however, not well known yet. Side effects were mild and rare.
A further meta-analysis of moderate- to high-quality RCTs (published in English) by Le and colleagues (2014) investigated the effects of rTMS specifically on hand function after stroke (as well as cortical excitability and any adverse events). Eight studies with a total of 273 patients were included; all subjects of the included trials had subcortical strokes.
Few adverse events were observed. The meta-analysis corroborated a positive effect of rTMS on finger motor ability (SMD 0.58, 95 % CI, 0.12–1.04; P = 0.01) and hand function (SMD −0.82, 95 % CI, −1.30 to −0.33; P = 0.0009). Changes of neurophysiological measures (MEP, RMT) by rTMS were not substantiated nor were motor performance changes for the unaffected hand (SMD −0.01) when the contralesional M1 was inhibited.
4.2.2.1 Best Evidence Synthesis and Its Relevance for Clinical Decision Making
What is the current state of the art regarding rTMS in motor rehabilitation after stroke?
A substantial number of RCTs have been published on the topic (compare Tables 4.1, 4.2, 4.3 and 4.4). Most address arm function, one gait. The data on gait rehabilitation is still so limited that while being reported above it will not be included in this best evidence synthesis that consequently will address arm function only.
Table 4.1
LF-rTMS of the contralesional motor cortex (LF-rTMS)
Reference | Study type and population | Intervention, comparison | Outcome measures, main results, conclusion |
|---|---|---|---|
Avenanti et al. (2012) | DB RCT, 30 subjects Chronic unilateral stroke sparing M1 Mild paresis | Real or sham rTMS before or after 45 min task-specific arm training 10 daily sessions, 1 Hz rTMS (1,500 pulses, 90 % RMT FDI), contralesional M1 | rTMS increased M1 excitability of the affected hemisphere, stable effect with rTMS-PT (3 months), gradual decline after PT-rTMS Bigger and more lasting improvements with dexterity after rTMS-PT compared to PT-rTMS Conclusion: rTMS-PT is more potent to enhance training-induced functional improvement among chronic stroke patients with mild motor impairment than PT-rTMS |
Conforto et al. (2012) | SB RCT 30 subjects Subacute (5–45 days) unilateral stroke (ICA) Hand paresis | Real or sham rTMS before or after 60 min training 10 daily sessions, 1 Hz rTMS (1,500 pulses, 90 % RMT APB), contralesional M1 | JTHF and pinch force improved only in the real rTMS group (inter-group difference n.s.); FM arm, MRS improvement in both groups (no inter-group difference) Conclusion: no additional benefit corroborated (underpowered study) |
RCT 60 subjects Subacute or chronic unilateral stroke (≥1 month) Mild to moderate hand disability | Real (1 Hz or 5 Hz) or sham rTMS combined with PT
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|



