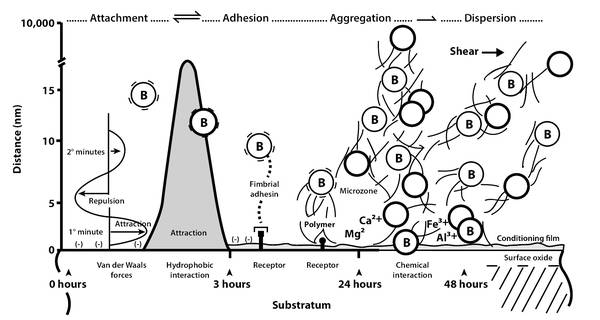
Intraoperative Complications A standard procedure in the surgical treatment of normal pressure hydrocephalus (NPH) is the implantation of a valve-controlled, ventriculoperitoneal (VP) shunt. The most frequent intraoperative complication during this procedure is incorrect positioning of the catheter. Older sources in the literature1 have reported a rate of up to 12% atypical positioning of the ventricular catheter depending on the site of the implantation. The most frequent incorrect positioning of the ventricular catheter was found for temporoparietal implantations, followed by implantations in the area of the central region and over the occipital lobes. The lowest rate of incorrect positioning was found for implantation of the ventricle catheter over the frontal brain. In contrast to the other three sites of implantation described, there were also the fewest neurological deficits as a result of incorrect positioning of a ventricular catheter over the frontal brain.1 By definition, in the above study, only primary incorrect positioning of the catheter was taken into account with regard to intraoperative complications and not secondary dislocations of catheters—for example, as a result of a gain in size or a secondary retraction of the catheter material with evidence of regular postoperative catheter positioning. Based on the above investigation, ventricular catheter implantation over a bore hole anterior to the coronal suture will be taken as the standard procedure. Incorrect positioning in the subcutaneous course of the catheter can result in the formation of slings, breakages, and disconnection, with consequential obstruction of cerebrospinal fluid (CSF) drainage. These disturbances can be caused by intraoperative and primary incorrect positioning, and also as a result of movement by the patient and growth in size. As a rule, incorrect positioning in the abdomen is associated with extraperitoneal catheter positioning, with CSF congestion, subcutaneous CSF accumulation, and signs of insufficient shunt function. Incorrect abdominal positioning as a result of perforation of the abdominal organs, the inguinal ring, and the navel is exceptionally rare, just as are injuries to the abdominal vessels and intraoperative injury to the intestines.1 In cases where it is not possible to drain into the peritoneum, conversion of atrial drainage represents a practical solution. Incorrect positioning of a ventriculoatrial (VA) shunt may be produced by a heart catheter that is too short or too long. This is a problem associated with the surgical technique. Intraoperative radiography and positioning of the tip of the ventricular catheter in the atrium, while strictly avoiding contact with the tricuspid valve, should be standard. In this context, injuries and bleeding from the veins of the neck and arteries, as well as the submandibular gland, the accessory nerves, and the vagus nerve, are very uncommon. Inadvertent implantation in the thorax with the formation of a hydrothorax is rare.1 However, in addition to the incorrect positioning of the ventricular catheter over the frontal bore hole anterior to the coronal suture, there may be other intracranial complications. Consequently, it is possible that intracranial bleeding may occur with a manual puncture as well as with a load-reducing-dependent puncture. The most frequent location of intracranial bleeding in this context is an intracerebral hematoma in the ventricular catheter. Marmarou et al2 reported a rate of up to 3% of intracerebral bleeding following shunt implantation. In our opinion, the rate of complications is around 1% to 2%. The causes of this kind of intracerebral bleeding can be hereditary (e.g., Waldenström disease), as well as through acquired bleeding disorders (e.g., alcohol abuse, coagulation-inhibiting drugs). Other forms of intracranial bleeding include subdural and epidural hematomas, which are rarely encountered. In contrast to the hematomas found in children, subgaleal and intragaleal bleeding occurs only rarely in adults. The risk of intraventricular bleeding from the choroid plexus exists only with revision interventions, when the ventricular catheter grows closely together with plexus structures and is removed only with substantial tension. At the same time, it is advisable to leave the ventricular catheter in situ so that there is danger of infection. Obstruction of the ventricular catheter opening during puncture, as a consequence of brain tissue detritus, has also been investigated scientifically. Utilizing a multicenter, prospective, and randomized study, researchers intended to demonstrate that a peel-away sheath could help eliminate this possibility of complications due to primary ventricle obstruction.3 Unfortunately, the “peel-away sheath” hydrocephalus study of contactless ventricular catheter insertion provided no statistically significant results.3 Consequently, no advantage was seen in the use of a peel-away sheath in the implantation of the ventricular catheter.3 Meier et al4,5 reported an incorrect positioning rate of 3.1% in a sample of 128 patients. In that study, primary incorrect positioning of the ventricular catheter (1.6%) occurred in two patients and, likewise, in two patients incorrect positioning of the abdominal catheter appeared in the abdominal skin or in an intra-abdominal cyst (1.6%). Sprung et al6 reported a rate of 6.9% incorrect positioning of the shunt catheter in a population of 144 patients; eight ventricular catheters (5.6%) and two peritoneal catheters (1.4%) were affected.6 Regarding rare complications (<0.1%) and very rare complications (<0.01%), there are only case reports and rates of these types of complications are not known (▶ Table 15.1).4,5,6 Intraoperative complication Rate (%) Catheter displacement 3–7 Ventricular catheter 1–6 Subcutaneous shunt 0–1 Peritoneal catheter 1–3 Intracranial bleeding (intracerebral hematoma) 0–3 Intestinal injuries Rare Unsuccessful catheter installation in the ventricle system, in the veins of patients with intracranial cysts, or in the peritoneum in patients with accretions Rare Injury to the large vessels of the abdomen Very rare Acute intraoperative pulmonary embolism Very rare Hydrothorax Very rare With intraoperative complications, the fundamental principle of neurosurgery—to avoid the same—still applies. However, this is not entirely possible for the incorrect positioning of a catheter. Some neurosurgeons7,8 tend to hold the view that the aid of neuronavigation or mechanical puncture can minimize the incorrect positioning of ventricular catheters. In the region of the peritoneal catheter, special trocars and/or laparoscopic implantation techniques perform the same task. However, it can be assumed that, by using these aids, a reduction in the rate of complications for “incorrect catheter positioning” can indeed be achieved, but it is unlikely to completely exclude this intraoperative complication. The authors suggest verifying the correct positioning of the catheter using postoperative computed tomography (CT) or magnetic resonance imaging (MRI) control of the head, which is considered to be an undisputed standard. The intra-abdominal position of peritoneal catheters should be documented using radiographic control of the abdomen at two levels when standing. In the event of incorrect positioning, the rule is to carry out a catheter revision as soon as possible when the patient is in a stable general condition. This is because incorrect positioning leads to obstructions in the shunt course in the majority of cases and can cause valve insufficiency, shunt insufficiency, and a negative course of the disease. In patients with bleeding or intracerebral hematoma with no clinical indications of an increase in intracranial increase, conservative therapy should be pursued first. At the same time, it is important that following the reabsorption of the bleeding, control of valve or shunt functionality must take place to control the blood that has entered the shunt because it can frequently block it. When shunt insufficiency has been demonstrated, the complete shunt, including the valve and the complete catheter, should be removed and replaced with a new system. All intracranial bleeding with an expansion effect and/or corresponding acute symptoms of an increase in intracranial pressure must be operated on immediately and be treated as a cerebral emergency. For this purpose, there is a vital OP indication. In rare cases of injury to the intestines or, very rarely, injury to the large abdominal vessels, the neurosurgeon should be advised to consult a visceral surgeon intraoperatively to clarify the situation as soon as possible. At the same time, the prognosis for the patient depends on the type of injury, its recognition, and correct specialist care. In this context, it should be assumed that, when there is an intestinal injury or injury to large abdominal vessels, the correct treatment should be given during the same procedure, and implantation of the peritoneal catheter should not take place in the abdomen. Consideration should be given as to whether the implantation of the peritoneal catheter should then take place in the interval or should be given precedence at another drainage site (atrium drainage). For patients who have already experienced revisional operations numerous times, this may prove to be difficult. In this case, a small venous diameter, strong adhesions or cysts in the abdominal cavity, as well as intracranial cysts may result in the need to use very infrequent drainage sites (e.g., intrapleural, intravesical). Guidelines from the various neurosurgical professional associations 2,8 give no recommendations regarding these rare and special drainage variant sites. Ay, think so still, till experience change thy mind. (Mephastophilis in Christopher Marlowe’s Doctor Faustus, c.1592) There are few data available in the literature on the subject of shunt infections in patients with idiopathic NPH (iNPH). As the epidemiologic parameters for these patients differ substantially from those for children who are the recipients of shunts, for whom many data are available, it is difficult to give specific evidence-based guidelines for iNPH. Nonetheless, the available literature on iNPH has been carefully reviewed and collated to describe the microbiologic mechanisms of shunt colonization. We propose that there are three categories of shunt-related infections, namely shunt colonization, shunt-related CSF infection/meningitis, and shut-related peritonitis/abdominal abscess. The colonization of VP shunts in patients with iNPH follows the same general mechanisms underlying the colonization of implanted biomaterials identified in the 1980s.10 To understand shunt infections, we also need to understand these mechanisms. Once a biomaterial such as a silicone catheter with a valve made of silicone or titan is implanted, it becomes an easy surface for colonization.11 The combination of traumatized tissue in the immediate vicinity of the implant and the lack of a host defense mechanism provides the ideal conditions for colonization by organisms. The initial attachment is determined by the physical characteristics of the cell, the fluid medium, and the surface of the material used, and can be characterized as reversible, nonspecific adhesion. At present we do not know whether the docking cell is a host cell of the patient or a polymerophil bacterium such as Staphylococcus epidermidis.12,13 The time window for attachment has been referred to as “the race for the surface.” Once the bacterial cell has adhered, short-range chemical interactions support the process. Nonspecific fimbrial interactions on the part of the bacteria now proceed in a manner like that of interactions with the glucoproteinaceous conditioning film that directly covers any artificial material brought into a mammalian body. The adhesion by exopolysaccharide glycocalyx polymers is irreversible. A continuous biofilm is now set up with microcolonies and single cells embedded in a highly hydrated, predominantly anionic matrix of bacterial exopolymers and trapped macromolecules.14 The ability to produce slime enables organisms to adhere to one another and to carry out an exchange of substances. The slime film acts as a mechanical barrier, protecting bacteria from host defense mechanisms and systemic antibiotics (▶ Fig. 15.1).15 Principles on how to avoid shunt colonization in patients with iNPH can be derived from the following knowledge. The source of bacteria that causes shunt colonization is normal skin flora. The local accumulation of possible infectious organisms in the vicinity of the implanted shunt results from: (a) direct contact between the skin and the shunt material before or during the implantation process, or (b) by immigration of the skin flora via the wound margin into the sites of shunt implantation during the surgical operation.16 The former can be avoided by using skin-covering film within the surgical wrap, while the latter can be avoided by increasing the speed of the operation, using just a few small surgical approaches to reduce surgical trauma, and adopting a “no touch policy”17 (i.e. opening the sterile wrap of the shunt catheters and the device as late as possible during the surgical procedure). A similar approach has been introduced for handling external ventricular drainages (EVDs).18 The time window, during which it becomes critical as to whether tissue cells or bacteria win the “race for the surface,”11 is known to be the few hours following the surgical procedure. Since the time window is known, we can apply prophylactic systemic antibiotics for its duration.19,20 Patients with a VP shunt can be infected with meningitis in the same diverse ways that patients without shunts can be infected. In patients with shunts, the shunt must be viewed as a potential for secondary complications because the infection is able to spread in an artificial way. On the other hand, a CSF infection or meningitis can, in and of itself, be a secondary complication in shunt patients because infections can spread into the CSF via the shunt. The most common manifestations of a CSF infection in patients with iNPH and shunts are slight or doubtful clinical signs of meningitis, such as minimal nuchal stiffness, slightly elevated temperatures, sleepiness, slightly elevated C-reactive protein (CRP) values, slightly elevated lactate values in the CSF, and slightly elevated protein values in the CSF. The microbiologic examination of the CSF will reveal the presence of an organism, which could possibly be a secondary contaminant, or it will show no contamination at all. Patients with iNPH can produce deceptive results. Because of their age, they do not tend to produce fulminant reactions of their immune system and can mislead the clinician into neglecting the signs of shunt-related CSF infections. Once they do show clear signs of such an infection, they have already been badly affected by the infection. Attention should be paid to abdominal symptoms in patients with a VP shunt. Patients can complain of abdominal pain and distension, dysuria, constipation, headache, and fever. Shunt-related abdominal infections can appear months or even years after the last shunt surgery. Consequently, the chronology may differ from a primary shunt colonization. A shunt-related abdominal pseudocyst, abscess, or peritonitis can be diagnosed using abdominal ultrasonography and/or CT scans. In patients with leukocytosis of the peripheral blood, no pleocytosis of the CSF and no clinical symptoms of meningitis, ligation of the shunt, and administration of systemic antibiotics can be performed. Kariyattil et al21 reported that abdominal symptoms may be the mode of presentation in patients with ascites, whereas shunt-related abdominal pseudocysts are more likely to present with shunt malfunction. Shunt-related abdominal abscesses can also appear with a time delay of months or years. Abdominal pain and fever associated with an elevated white cell count are typical. Ultrasonography studies and enhanced CT scans usually show well-defined, lobular fluid collections. Methicillin-resistant Staphylococcus aureus, Proteus mirabilis, and Staphylococcus epidermidis have been implicated as the organisms causing shunt-related abdominal abscesses.22 Surgical revision is essential in the event of shunt-related abscesses. The shunt has to be removed and externalized until the infection has been eradicated with certainty. The frequency with which shunt infections are detected and the organisms are identified depends to a large extent on the methods used.23,24,25,26 Most authors do not describe the pathway of infection in patients with suspected shunt infection when the focus of the paper is not shunt infections per se. Indeed, in some publications, the rate of shunt infections has not been reported at all. The rule of thumb is: the more conscientious authors are, and the longer the follow-up period is, the higher is the reported rate of shunt infections. Otherwise, the more well established the diagnostic pathway, the more precisely defined are the criteria to be met in diagnosing an iNPH, and the lower the rate of shunt infections. Reviewing the literature related to shunt infections in patients with iNPH over the last 20 years, we have become aware that a realistic rate of shunt infection in a follow-up period of 2 years or less is about 3% to 6%. In long-term follow-up periods of 5 years’ duration or more, 10% is a realistic rate. However, we have to consider the fact that peritonitis and meningitis occur independently of the presence of VP shunts. The incidence of these infections has been unavoidably included in the infection rates given in publications reporting long-term follow-up (▶ Table 15.2, ▶ Table 15.3). Author Year of publication No. of patients Follow-up time Infection rate (%) Mirzayan et al80 2010 34a 80.9 ± 51.6 months 8a Meier et al81 2008 148 12 months 3 McGirt et al82 2008 132 18 ± 13 months 12 Kahlon et al83 2007 27b 5.5 ± 1.4 years 3b Marmarou et al84 2005 102c 12 months 3 Sorteberg et al85 2004 17 9 (5–15) months 6 Boon et al86 1998 96 12 months 3 Larsson et al30 1991 74 2.1 years 19 Greenberg et al35 1977 45d 16.7 (3–29) months 7 a34 patients with a long-term follow-up, out of a total of 51 treated patients; the infection rate was given for the total number of patients (4 of 51). b27 patients with a long-term follow-up, out of a total of 75 treated patients; the infection rate was given for the total number of 75 patients (1 patient with shunt infection, 1 patient with wound infection). c102 patients of a total of 151 treated patients underwent shunt surgery. d45 patients with follow-up >12 months, out of a total of 73 treated patients. Abbreviation: iNPH, idiopathic normal pressure hydrocephalus. Author Year of publication No. of patients Follow-up time Infection rate (%) Eide and Sorteberg87 2010 130 2 (0.3–6) years 9 Pujari et al88 2008 55 5.9 ± 2.5 years 10 Zemack and Romner89 2002 147 26.7 months 6.4a Lund-Johanson et al40 1994 95 1–9 yearsb 8.4 aOf a total 218 patients, including 71 patients with secondary normal pressure hydrocephalus. bNo mean follow-up time given. Abbreviation: iNPH, idiopathic normal pressure hydrocephalus. Chang et al27 reported on the management of 32 patients with NPH by insertion of a lumboperitoneal (LP) shunt, and found one patient (3%) with a shunt infection 4 months after surgery. There are almost no current data available regarding infection rates in VA shunts for iNPH. Bret et al28 needed to perform repeat operations as a result of septic complications in 5% of a patient collective of 129 patients, including 14 patients with a VA shunt, within a follow-up period of 16.7 months. The organisms found responsible for shunt colonization originate, in the majority of cases, from skin flora.29 Larsson et al found that CSF cultures grew Staphylococcus epidermidis in 68%, Propionibacterium acnes in 12%, and were negative in 18% of cases of clinical infections in patients with NPH.30 Fan-Harvard assumed that infections of central nervous system shunts are dominated by coagulase-negative staphylococci, with Staphylococcus epidermidis accounting for 50% to 75% of infections, followed by Staphylococcus aureus.30 Walters et al assessed31 200 pediatric cases with VP shunt infections and found gram-positive cocci distributed to Staphylococcus epidermidis in 47%, Staphylococcus aureus in 27%, Streptococcus faecalis in 10%, and miscellaneous in 13%, as well as gram-negative rods: Escherichia sp. 19%, Klebsiella sp. 19%, Pseudomonas sp. 8%, and miscellaneous 4%. According to Bayston et al,32 Propionibacterium acnes, a normal anaerobic skin inhabitant, causes up to 14% of infections. Livni et al33 isolated Staphylococcus epidermidis and Staphylococcus aureus from infected shunt material. Sandoe and Longshaw34 were able to show that Staphylococcus lugdunensis caused a VP shunt infection. The clinical presentation of patients with shunt infections can occur with obvious symptoms leading to the diagnosis. Fever, along with rubor of the track of the shunt, will be relatively straightforward symptoms. However, the vast majority of patients will present with less straightforward symptoms or mild symptoms, or they will present with symptoms that could allow different diagnoses. The period of time between surgery and the occurrence of primary shunt colonization is, in our experience, between a few days to about 2 months. Shunt-related infections occurring beyond 2 months after surgery almost always have other causes than the surgery itself. In these cases, we have to look for a reason, which can fall into one of three categories: Mechanical cause (e.g., secondary exposition of shunt or valve due to permanent pressure of the temples of spectacles onto a retroauriculargly implanted valve) Primary infection of any of the body compartments penetrated by the shunt due to factors not dependent on hydrocephalus and the shunt Changes in the immunocompetence of the patient due to factors independent of hydrocephalus and shunt that result in an outbreak of a clinically nonevident shunt colonization acquired during surgery There do not appear to be any published studies in which a differentiation has been made between different types of shunt-related infections. Reports in the literature have given a wide range of times between surgery and shunt-related infections developing in patients with iNPH, for example, 1 to 30 weeks30 and 1 to 14 months.35 These data suggest that several types of infections have been recorded. A special subgroup of shunt infections consists of delayed infections caused by long-term changes in the material used for the shunt system itself, such as mineralization and biodegradation of silicone materials. These changes are well known in pediatric patients but are not a serious problem in patients with iNPH.36 In addition to the neurologic examination of patients suspected of having a shunt-related infection, a local examination and palpation of the shunt are needed. Wound dehiscence, sutures that stick out, exposed parts of the shunt, and rubor along the shunt trace, in particular, should be looked for. The abdomen should be palpated and auscultated. The neurologic examination should target symptoms of meningitis, as well as symptoms of shunt dysfunction, especially underdrainage due to abdominal cysts or obstruction by infectious concrement. There are types of sample to obtain for a paraclinical examination: serum samples, CSF samples, and parts of the shunt (e.g., catheter tips). Obtaining the latter involves the explantation of the shunt. The analysis of serum CRP and leukocytes has not been reviewed for patients with iNPH, but it has for other patients with shunts. Schuhmann et al37 noted that measuring CRP in blood serum significantly increased the precision of diagnosis of a shunt infection. CSF samples can be obtained from a valve reservoir or via a lumbar puncture. The advantage of CSF samples from the valve reservoir is that they are taken from an area with a high CSF turnover and, therefore, the CSF lactate and protein content, and number of cells change relatively rapidly with changes in infectious events. Compared with CSF from the valve reservoir, these parameters may show changes for a long period of time after an infectious event has occurred if taken from a lumbar puncture. The disadvantage of puncturing the valve prechamber is the danger of a de novo infection caused by the puncture. Every time a CSF sample is taken, material for microbiologic examination should also be taken. The examination of parts of the shunt should not be considered to be a regular part of the diagnostic process, as it can only be performed if the surgeon has already explanted the shunt as a result of a serious suspicion of shunt-related infection. Several authors tend toward making an “overdiagnosis” by examining parts of the shunt apparatus. Bayston et al38,39 reported positive cultures on explanted shunt parts in the absence of clinical infections. Walters et al31 compared the clinical presentation with microbiologic examination of CSF samples and microbiologic examination of the shunt apparatus. They found a precise match only in cases in which there were meningeal signs of infection. In patients with fever alone or peritoneal signs alone, CSF cultures were less sensitive than cultures of parts of the shunt. Shunt infections are a source of frustration for the surgeon and dangerous for the patient. Therefore, the avoidance of shunt infections in patients with iNPH should be a priority. There are three possibilities of prevention associated with the surgeon, the patient, and the material. Lund-Johansen et al40 found that the infection rate was higher among patients operated on by residents, whereas the choice of the shunt type (Orbis-Sigma, Holter, Hakim) and the perioperative use of antibiotics were not correlated with complication or failure rates. As the proviso to let only experienced surgeons implant shunts would work, at the very most, for one generation only, we have to further differentiate the factor “surgeon.” Risk factors for shunt infections associated with the surgeon are extended operation time, subcutaneous hematoma as a culture medium for bacteria, provoking extensive skin contact with the shunt material, and subcutaneous sutures that are too long and cause suture granuloma. The probability of contamination of the shunt material with organisms originating from the surgeon seems to be extremely low; in a swab series, Bayston et al demonstrated that organisms involved in shunt colonization were present on the patient preoperatively.41,42,43,44,45 Another risk factor associated with the surgeon is the choice of an inadequate shunt system, which can result in the need for revision surgery and thereby increase the risk of infection. In our patients with iNPH, we found a correlation between the rates of shunt infections and the presence of diabetes mellitus, adiposity, decubitus ulcer, and raised CRP or leukocytosis. Because the first two cannot be cured in a short period of time, these patients should be operated on by experienced surgeons to reduce other risks of shunt infections. In cases of pre-existing infections, with increased levels of CRP or leukocytosis, as well as clinically detectable infections without increased levels of these parameters, shunt insertion should be postponed by at least 4 weeks. Bayston et al46,47 were able to show that a high CSF protein content per se does not increase the risk of shunt infections. Brydon speculated that the risk of CSF infections in patients who are hyperproteinorrhaghic might be increased because they have a different skin flora, with possibly more pathogenic organisms present.46,47 The alternatives that can be used to prevent shunt infections in patients with iNPH by selecting an appropriate implant material can be divided in two categories, which are described below. The first category includes all possibilities for avoiding later indications requiring revision of the shunt system due to complications other than infections. Programmable valves can help avoid reoperations due to overdrainage or underdrainage and thereby avoid infectious complications. CSF reservoirs enable the surgeon to confirm the suspicion of a shunt infection and to avoid a needless explantation of noninfected shunts. Therefore, we would recommend that programmable valves with reservoirs should be the standard alternative to be used in the treatment of iNPH. The second category involves the use of antibacterial or bacteriostatic shunt materials. Materials using two different principles are available. One is the impregnation of the silicone shunt material with antibiotic substances, while the other uses the impregnation of the silicone with silver nanoparticles. BACTISEAL (Codman, Johnson & Johnson, Raynham, Massachusetts, United States) was the first material available. BACTISEAL is a silicone rubber impregnated with clindamycin and rifampicin. The effectiveness of the material has been shown both in vitro and in vivo.14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54 Even when the antimicrobial-impregnated silicone shunt material was experimentally covered with a plasma protein conditioning film and exposed to Staphylococcus epidermidis, it was able to kill the adhering bacteria within 48 to 52 hours.43 Early fear that BACTISEAL catheters shows a risk of epileptogenic potential has not been substantiated.55 There is disagreement in the literature regarding the in vitro results for SILVERLINE catheters (Spiegelberg, Hamburg, Germany). Bayston et al52 reported that SILVERLINE catheters do not show the ability to eradicate higher inocula of contaminating bacteria during in vitro tests, while other authors have reported low bacterial colonization during in vitro experiments.50 Nonetheless, there is evidence that SILVERLINE catheters work in practice. Lackner et al56 found a significantly lower rate of catheter-related ventriculitis in patients with occlusive hydrocephalus. Izci et al57 implanted VP shunts with SILVERLINE ventricular catheters in seven patients with a positive CSF culture and reported that the CSF culture became negative within a period of 14 months. In our patient sample, we were able to prove the effectiveness of SILVERLINE EVDs in relation to the prevention of catheter-related CSF infections.58 There is no evidence demonstrating a toxic risk of SILVERLINE catheters, although silver sulfide deposits have been identified in the tissue, and silver was released into the CSF.59,60 The type of treatment suitable for shunt infections depends on the type of shunt-related infection. As we discussed in Section ▶ 15.2.1, three categories of shunt-related infections can be differentiated: shunt colonization, shunt-associated meningitis, and shunt-related peritonitis. Shunt colonization originates from biofilm development following the contamination of the shunt material with skin flora and the subsequent victory of the skin flora in the “race for the surface” against the tissue cells. Shunt colonization can be primary—on implantation of the shunt—or secondary—taking place at a specific event (e.g., exposure of the shunt as the result of a skin defect). In both cases, bacteria have already won the “race for the surface” and there is no possibility of contesting the victory. Therefore, any shunt that has become colonized must be removed. If a shunt-related infection occurs within the first 2 months following implantation, then we have to assume that shunt colonization has taken place. If a shunt-related infection occurs in the context of an exposure of the shunt or valve—independent of the time that has elapsed since the shunt was implanted—then we also have to assume that colonization of the shunt has taken place. Despite the fact that there are reports that shunts remain exposed for a long period of time (e.g., 15 months), every confirmed case of colonization of a shunt results in an infection of the CSF sooner or later.61 Adhesion-mediated infections develop that are notoriously resistant to antibiotics and host defenses, and tend to persist until the biomaterial or foreign body is removed.11,62 In the 1980s, during which time different treatment modalities were advocated in neurosurgery, James et al63 conducted a randomized study of patients with a shunt-related infection using three treatment groups. Group A underwent shunt removal and received systemic antibiotics and external ventricular drainage for the administration of antibiotics. Group B was treated with removal and immediate replacement of the shunt, and intrashunt antibiotic therapy. Group C received antibiotics without removing or replacing the shunt. All patients in Group A and 90% of the patients in Group B were treated successfully, whereas only three patients in Group C responded to treatment. Walters et al confirmed these results in a retrospective analysis of more than 200 patients.64 Therefore, we recommend removal of the shunt in patients in whom there is a reasonable suspicion of shunt colonization, even if there are no signs of a CSF infection, meningitis, or generalized infection. In patients with iNPH where there is colonization of a shunt but no signs of CSF infection, and if the patient tolerates being without a shunt for a period of about 3 months, an EVD is not implanted. The patient should be treated with intravenous antibiotics, primarily to prevent the spread of the infection to the CSF. With regard to the infection per se, the host of the bacterial load is removed with the shunt, and the antibiotic therapy has, in some way, a “preventive” characteristic. Therefore, there is no need to take a sledgehammer to “crack a nut.” Recommendations regarding modern broad-spectrum antibiotics (e.g., linezolid) for the treatment of shunt-related infections can be found in the literature.65 In our opinion, second-generation cephalosporins that permeate the CSF are adequate for most patients. We would recommend application for 7 to 10 days after removing the shunt. The implantation of the new shunt should be carried out about 3 months after the removal of the old one. We prefer to use the opposite side for implantation of the new shunt. If the hydrocephalic constellation of the patient necessitates permanent drainage after removal of the shunt, then we recommend the implantation of an EVD (SILVERLINE or BACTISEAL) on the same side of the shunt that has been removed. In addition, systemic antibiotics should be given. Intrathecal administration of antibiotics is not necessary in patients who do not have symptoms of meningitis. Following systemic antibiotic therapy over a period of 7 to 10 days and a 3-day period with no antibiotics, bacteriologic tests are carried out on a sample of CSF. If the sample is sterile and serum parameters (CRP, leukocytes) indicate no systemic inflammation, then a new shunt (SILVERLINE or BACTISEAL) can be implanted on the opposite side. It should be stressed once more that this situation is an exception in patients with iNPH. It is very uncommon to find meningitis in a patient who has had a VP shunt for iNPH. In such cases, the shunt should be removed and an EVD (SILVERLINE or BACTISEAL) should be implanted on the opposite to the side of the shunt. Bacteriologic testing of a CSF sample taken perioperatively should be carried out. Directed systemic antibiotics can be started immediately and intrathecal antibiotics should be initiated once the results of bacteriologic tests have become available. Our experience is limited to the off-label use of vancomycin (2 × 5 mg intrathecal). We generally give this for 10 days. After 48 to 72 hours after intrathecal application of antibiotics has ceased, we take a CSF sample for bacteriologic tests. If this sample is sterile and serum parameters (CRP, leukocytes) indicate no systemic inflammation, then the EVD is removed. A new shunt (SILVERLINE or BACTISEAL) can be implanted on the opposite side after about 3 months. The development of a cerebral abscess around the ventricular catheter of a VP shunt in patients with iNPH is very uncommon. We do not have experience of our own in this regard; however, in the literature, one can find recommendations for the neuroendoscopic removal of both the ventricular catheter and the abscess.66 Recurrent pleural effusions have been reported in patients with shunt-related infections in ventriculopleural shunts. These patients also show high lactate dehydrogenase levels and lymphocytosis in the pleural fluid. Shunt ligation has been recommended as treatment.67 Unlike other patients with a shunt and peritonitis, those with iNPH will overcome in 4 to 6 weeks with no CSF drainage. Consequently, the procedure of the first choice should be to remove the entire shunt system and reimplant a new shunt system after the completion of the treatment of peritonitis with antibiotics. In cases in which there is some doubt regarding an abdominal infection, or if sterile inflammation of a dislocated abdominal catheter is more likely, one option may be to ligate the shunt on the downstream side of the valve (e.g., subclavicular) and remove only the abdominal catheter to replace it after antibiotic treatment. Most cases that were tried to treat in this way, for whatever reason, ended with the removal of the entire shunt at an earlier or later stage. The successful conversion of VP shunts into VA shunts in cases of clinically isolated abdominal shunt-related infections has been reported in the literature, but they have been of an experimental nature only.68 Complications in shunt surgery can be divided into surgical complications and valve-related or shunt-related complications. Surgical complications, which account for approximately 50% or more of all complications, include early infection, wound dehiscence, all types of disconnection, and misplacement of shunt parts (e.g., proximal ventricular catheter, valve, distal peritoneal or atrial catheter; ▶ Fig. 15.2, ▶ Fig. 15.3a, ▶ Fig. 15.3b). Fig. 15.2 Secondary dislocation of the distal (peritoneal) catheter.
15.2 Infections
15.2.1 What is a Shunt Infection?
15.2.2 Shunt Colonization
Biofilm Development
Clinical Implications of Biofilm Development
15.2.3 Shunt-Related CSF Infection/Meningitis
15.2.4 Shunt-Related Peritonitis/Abdominal Abscess
15.2.5 Epidemiology of Shunt Infections in Patients With iNPH
Frequency of Shunt Infections
Lumboperitoneal/Ventriculoatrial Shunts in NPH
Organisms
15.2.6 Does My Patient Have a Shunt Infection?
Clinical Diagnosis of Shunt Infections
What Can We Learn From the Time of Outbreak of a Shunt Infection?
Clinical Examination
Paraclinical Examination
15.2.7 How Can Shunt Infections Be Avoided in iNPH?
The Surgeon
The Patient
The Material
BACTISEAL
SILVERLINE
15.2.8 How Can Shunt-Related Infections Be Treated?
Treatment of Shunt Colonization
Treatment of Meningitis in Patients With a Shunt
Treatment of Peritonitis in Patients With a Shunt
15.3 Postoperative Complications
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree