Fig. 12.1
Hypertrophy of the right cardiac cavities with right deviation of the heart in a child with a VAS positioned at T4 level (arrow) who developed pulmonary embolism and hypertension

Fig. 12.2
Electrocardiogram showing the signs of right hypertrophy and right deviation of the cardiac axis in a patient with cor pulmonale
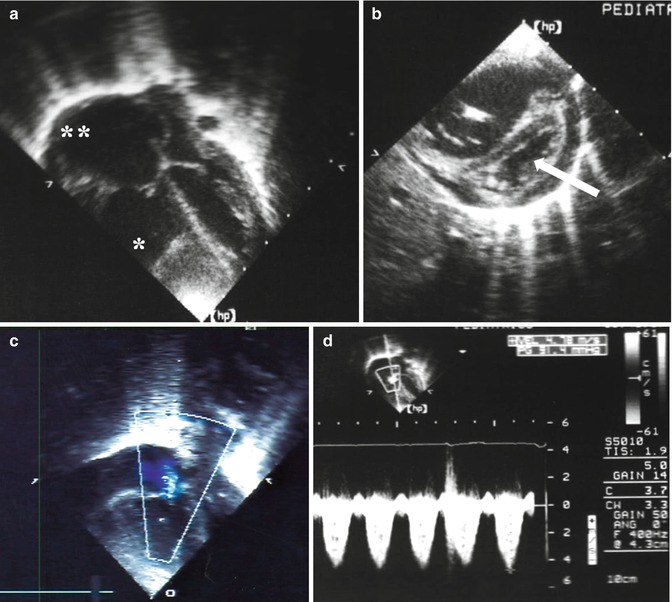
Fig. 12.3
Echocardiographic signs in a case of pulmonary hypertension: (a) severe enlargement of the right ventricle (asterisk) and atrium (double asterisk); (b) hypertrophy of the right ventricle with shift of the ventricular septum (arrow) toward the left ventricle; (c) blood regurgitation through the tricuspid valve; (d) pulmonary hypertension: the overall value is about 100 mmHg resulting from the gradient through the tricuspid valve (91.4 mmHg) plus the atrial pressure (10 mmHg)
The surgical management consists of the removal of the VAS, to be performed as early as possible and to be converted into VPS or other shunts (e.g., ventriculo-gallbladder or ventriculo-bladder shunt), or endoscopic treatment. The medical treatment is first based on pharmacological thrombolysis, then on diuretic and anticoagulant drugs. Medical therapies including vasodilators and prostacyclin analogues can be used in the attempt of reducing the pulmonary vascular resistance [3]. Unfortunately, the prognosis of pulmonary hypertension complicating VAS is often dismal, with a 50–100 % rate of mortality [28, 39]. Reduction of the risk of shunt colonization, elective revision of the shunt if a migration into the superior vena cava is detected, periodic checkup screening to exclude atrial thrombosis or initial signs of pulmonary hypertension (chest X-rays, electrocardiography, echocardiography), and, according to some authors [35], anti-aggregation drug prophylaxis, can be attempted to reduce the risk of thromboembolic complications.
12.2.2 Thrombosis
The formation of clots inside the right atrium, possibly extending to the pulmonary artery and/or the superior vena cava/internal jugular vein, is a quite common complication of VAS. Actually, the frequency on autoptic series ranges around 60–100 % [9]. However, the clinical evidence is lower, ranging from 2 to 50 % [37]. The phenomenon is macroscopically described as fibrinous material with attached clots surrounding the tip of the atrial catheter [13]. The clot is usually attached to the atrial wall, with a portion floating free.
The pathogenesis and the consequences of atriovenous thrombosis are reported in the previous paragraph. In addition, the risk of enlargement of the thrombus up to the intracranial sinuses or the tricuspid valve has to be taken into account (Fig. 12.4). This phenomenon is also favored by remnants of the distal catheter left in place for a long time because they are undetected or hard to remove [7]. The diagnostic management consists of chest X-rays and echocardiography, completed by transesophageal ultrasounds, D-dimer test, and high-resolution CT scan, to confirm the diagnosis and to exclude pulmonary embolization. Moreover, it is important to rule out factors inducing thrombophilia (e.g., homozygous factor V Leiden mutation or intake of oral contraceptives). The treatment includes anticoagulation therapy, antibiotic prophylaxis (to prevent endocarditis), and removal of the shunt. A thoracocentesis may be required to confirm the diagnosis or in the case of abundant pleural transudate [37].


Fig. 12.4
Angio-CT scan of the brain showing a filling defect of the right jugular bulb (arrow) in a patient whose VAS was removed because of jugular/vena cava thrombosis
12.2.3 Endocarditis
Endocarditis in VAS occurs as a result of the combination of atrial thrombus and bacteremia [43]. Indeed, the clinical history usually discloses previous, recurrent infections (e.g., urinary tract or bronchial tubes). Fever, asthenia, skin purpuric rash, and presence of cardiac murmur are the main clinical signs/symptoms. It is worth noting that the cardiac murmur can be poorly appreciated or even absent in the early phases of infective endocarditis [6]. The diagnostic work-up includes transthoracic and/or transesophageal ultrasounds, which show the typical atrial floating vegetation, and blood examinations and cultures, which demonstrate the signs of bacteremia or sepsis. The cultures from all the possible sites of contamination have to be obtained to identify the primary site of infection.
The management of VAS-related endocarditis is based on: (1) prompt antibiotic therapy administration, to hinder the infection; (2) anticoagulant treatment, to prevent pulmonary embolization; (3) removal of the shunt, to eliminate the source of the thrombosis and/or the infection; and, if needed, (4) removal of the atrial clot by cardiac surgery, to prevent tricuspid obstruction and/or pulmonary embolization (especially if the atrial clot is detached after the neurosurgical removal of the VAS) [6].
12.2.4 Cardiac Tamponade
This is a rare, late complication of VAS resulting from the progressive erosion of the myocardium leading to atrial perforation and pericardial effusion. In the series of 455 patients with VAS reported by Forrest and Cooper, cardiac tamponade occurred in three cases (0.6 %) [16]. The authors noticed that, in two out those three patients, the atrial erosion was probably due to an undesirable position of the tip of the atrial catheter, which was entangled in the pectinate muscles of the right atrium. Actually, the perforation of the myocardium is thought to result from an increased stiffness of the tip of the atrial catheter, due to its abnormal position or to a clot filling and stiffening it [9]. Exceptionally, the myocardial damage can be provoked intraoperatively by the forceful introduction of the atrial catheter with a stylet into a thrombotic jugular vein [36]. In all these instances, the cardiac tamponade is related to the blood pericardial effusion due to the bleeding from the perforation site. However, some unusual cases of tamponade due to the CSF accumulating into the pericardium and coming from a migrated atrial catheter perforating the cardiac walls have been described [12, 23]. Mastroianni et al. even reported on a 48-year-old woman whose pericardial tamponade resulted from a disconnected remnant of VAS perforating the right ventricle and draining CSF, thanks to a fibrin sheath still connecting it with the proximal part of the shunt [27].
Cardiac tamponade is a life-threatening condition that requires an emergency management to prevent low cardiac output and cardiac arrest. Dyspnea, respiratory distress, tachycardia, swelling of the jugular veins, softened hear sounds, and, finally, signs of shock are the most common clinical signs. Chest X-rays show an enlarged pericardial shadow and clear lung fields, while chest CT scan can also demonstrate a pericardial and pleural effusion other than the position of the shunting catheter. Echocardiography points out the circumferential pericardial effusion with a compression of the right heart cavities during the diastole (Fig. 12.5). Laboratory signs of multiorgan failure can be appreciated in the late phases. The patients are monitored and stabilized in an intensive care unit; then, a pericardiocentesis or, if this is unfeasible or ineffective, an open cardio-surgical (sternotomy) evacuation of the pericardial effusion with surgical repair of the atrial or ventricular perforation and extraction/replacement of the shunt are performed.


Fig. 12.5
Cardiac tamponade in a young child because of severe circumferential pericardial effusion (asterisks)
12.2.5 Shunt Nephritis
This complication is a result of the colonization of the VAS by a low-virulence microorganism. The chronic infection is usually sustained by Staphylococcus epidermidis or, less frequently, by Staphylococcus aureus, Propionibacterium acnes, Listeria monocytogenes, and Pseudomonas aeruginosa [9]. Such a persistent infection induces an immune-complex disease through a chronic hyperantigenemia and hyperglobulinemia with deposition of complements, immunoglobulins, and immune complexes on the glomerular basement membranes [39]. In more detail, the persistent antigenemia due to the long-lasting immunization of the host against the low-virulence bacterium (the bacterium proliferates by adhering to the shunt and continuously releases its antigens) leads to the continuous formation of antigen-antibody complexes in antibody excess, with secondary activation of the complement system and deposits in the glomerular capillary wall, cytokine release, and subsequent membranoproliferative or focal proliferative glomerulonephritis. The histologic analysis of autoptic kidney specimens actually points out mesangial cell proliferation with widening of the mesangial matrix, granular deposits, and thickening of the glomerular basement membrane [26]. The immunofluorescence observation of the deposits reveals the presence of bacterial antigen, complements, IgM, IgG, and fibrinogen [44].
Shunt nephritis is a rare, usually late complication, occurring several months/years after the VAS placement [38]. Infants and children are more prone to develop it than adolescents and adults because of the higher risk of shunt infection by coagulase-negative staphylococci. The early clinical picture is characterized by fever, anemia, hepatosplenomegaly, and signs of septicemia (including positive blood cultures). Afterward, a nephritic syndrome (arterial hypertension, proteinuria, azotemia) or, less commonly, a nephrotic syndrome appears (severe proteinuria, hypoproteinemia, body edema). The diagnosis is obtained by demonstrating the renal impairment, with a glomerular filtration rate decreased up to 20–45 ml/min, associated with hypocomplementemia and high serum levels of cryoglobulins and bacterial antibodies. Shunt removal and the antibiotic therapy are generally able to stop the nephritis and to restore a normal renal function in most cases; otherwise, immunosuppressive drugs can be added in the severe or refractory forms. It has been sporadically observed that the complement activation is not interrupted by the shunt removal and replacement, thus suggesting the persistence of the antigen somewhere (e.g., inside the ventricles) as activating factor [41]. Shunt nephritis can be prevented by periodic blood examinations aiming at monitoring the renal function and, in suspected cases, the C3 and C4 levels. The prevention of this complication is mandatory because, though rarely, fatal cases have been reported [44].
12.3 Complications Shared with VPS
12.3.1 Septicemia
Septicemia is the most common complication of VAS, its frequency ranging from 10 to 15 % of cases [5]. According to the review by Luthardt on 1540 published cases during the VAS era, its incidence was actually 13.5 % [25]. Septicemia also accounts for the highest rate of mortality among VAS complications, especially in infants and/or immunodepressed patients [10]. Indeed, sepsis in VAS is complicated, other than by the multiorgan failure due to the action of the microorganism, also by the possible occurrence of shunt malfunction and thrombosis around the cardiac catheter followed by the aforementioned cardiopulmonary complications. Once again, coagulase-negative staphylococci are the most frequently involved in the infectious process [2]. Staphylococcus aureus is usually associated with a highly virulent and widely diffused infection so that the clinical course may be acute or fulminating. Differently, Staphylococcus epidermidis, which is also the most frequently involved bacterium, shows a more indolent and chronic course. The clinical picture is characterized by fever, signs and symptoms of progressive anemia, lethargy, splenomegaly, and, later on, petechiae, hemorrhages, and multiorgan failure. Laboratory investigations point out leukocytosis and positive blood cultures. Bacteremia in infected VAS is more frequently detected than in colonized VPS [33].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








