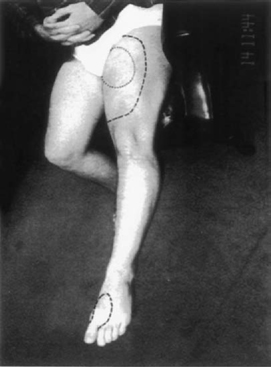
21 Conservative versus Surgical Treatment and Prognostic Evaluation for Tethered Cord Syndrome Shokei Yamada, George T. Mandybur, Austin R. T. Colohan and Vivian A. Yamada This chapter summarizes the previous chapters and reflects on the neurosurgeon’s role in the diagnosis and treatment of tethered cord syndrome (TCS). Given that the presentations in the previous chapters clarified the current understandings, the combination of the neurological and musculoskeletal findings and imaging criteria can lead to the diagnosis of TCS. However, decisions about treatment for TCS are more complicated. Once the diagnosis of TCS is established or when there is an established likelihood that TCS is evolving, the neurosurgeon is required to choose among treatment alternatives that will help patients maintain normal lives while minimizing surgical risks. Additional information about TCS related to various other anomalies and disorders is provided to supplement the previous chapters. Embryology emphasizes the importance of neurulation and caudal mass regression. The position of the caudal extremity and the development of a normal filum determine the healthy lumbosacral spine and spinal cord. The failure of these two components to develop properly can cause TCS (Chapter 2). The indication and surgical treatment must be definitive in patients with elongated spinal cord attached to inelastic structures, including a fibrous filum, lipoma, or dermoid, and with neurological deficits and musculoskeletal deformities in the lower limbs (Chapter 10). The pathophysiology of the tethered spinal cord or TCS includes impairment of oxidative metabolism and electrophysiological function (Chapter 3). Mild to moderate metabolic dysfunction caused by low- or medium-grade traction of the experimental cord is reversible. The degree of dysfunction may be related to spinal cord ischemia and corresponds to a mild or moderate form of neurological deficit observed in humans. No histological damage to neurons, glial cells, or vasculature is expected in the spinal cord with such metabolic and electrophysiological dysfunction. However, sudden traction, in addition to steady cord traction, can cause histological damage to neurons. These findings may explain the permanent but partial damage to the lumbosacral cord, particularly to the conus medullaris (Chapter 3). Neurological and urological examinations must include detailed history taking, specific for the early signs and symptoms of TCS. Neurological signs and symptoms (Chapters 4, 10, and 15) and urological tests (Chapters 7 and 8) must be correlated with lumbosacral lesions above the cord-tethering site. Skin stigmata can be useful when correlated with signs and symptoms and imaging features for diagnosis of TCS (Chapter 10), but older patients often lack these features. Both autonomic and somatic systems form afferent and efferent reflex arcs, regulate the complex urinary storage and emptying functions, and also participate in the urethral control mechanism. The incontinence in TCS is due to the dysfunction of neurons located within the conus medullaris.1 A full urodynamic evaluation along with pelvic floor electromyography (EMG) plays an important role in detecting a conus medullaris lesion or sacral neuropathy in patients suspected of having TCS (Chapter 7 and 8). Determination of the postvoid urinary residual for these patients at the clinic is a practical method of detecting early TCS. These methods are valuable in differentiating incontinence due to TCS from psychological incontinence and incontinence due to bladder infection. Imaging studies have improved the diagnosis of TCS since the advent of computed tomography (CT) and magnetic resonance imaging (MRI). CT or MRI evidence of an elongated cord continuous to a thick filum or a tumor predicts TCS (Chapter 5). For adolescent or adult patients without these MRI features, a posteriorly displaced filum is a useful diagnostic feature for TCS (Chapter 15). To confirm this finding, intraoperative endoscopy confirms the displaced filum posterior to the cauda equina fibers and touching the posterior arachnoid (Chapter 3). In infants with TCS, ultrasonic studies can demonstrate lack of filum and conus movement, which is supposed to be synchronous to cerebrospinal fluid pulsations in normal individuals (Chapter 6). TCS in the cervical spinal cord (Chapter 9) is clinically manifested like category 2 of the lumbosacral TCS (Chapter 3). The spinal cord lesion is located within a few segments above or below the tethering point because the dentate ligaments hold the cord tightly to prevent stretch-induced cord dysfunction. However, there is experimental evidence that cephalad pulling of a pair of cervical dentate ligaments causes elongation of the cervical cord and extending down to the thoracic cord segments.2,3 This supports the fact that cervical TCS can really exist. The mechanical causes of tethered cervical spinal cord syndrome include myelomeningoceles (MMCs), lipomyelomeningoceles, and dermoid or postoperative scar formation. Chapter 14 concentrates on TCS associated with a dermoid or epidermoid tumor. It emphasizes the elimination of the cord tethering effect of a tumor as well as decompression of the cord mass effect. Total resection must be accomplished, although two-staged operations may be required for a large tumor. In addition, an intradural lipoma and dysgenesis of the spinal cord or peripheral nerves must be considered. Chapter 20 discusses evoked potential studies in conjunction with clinical findings in true TCS [i.e., category 1 cord tethering (Chapter 3)]. The authors found delayed conduction from the spinal cord to the cervical region and cerebral cortex in response to posterior tibial nerve stimulation. This delay is interpreted as impairment of multisynaptic spinal cord conduction, and the postuntethering improvement in evoked potentials is significant. Commonly, straight conduction through the posterior column is not disturbed in TCS patients. Only in category 3 is it likely that the lumbar and cervical somatosensory evoked potentials may be absent, under severe cord tethering or peripheral nerve dysfunction. While making treatment decisions, neurosurgeons and other specialists must take into account that the prognosis of TCS patients or those harboring potential cord-tethering disorders depends on several individualized factors. Among these factors are: (1) neurological signs and symptoms must be precisely described (Chapter 4, Chapter 15). (See an example of sensory changes with patchy distribution in Fig. 21.1.) (2) The severity of TCS signs and symptoms determines the prognosis; inelastic structures that are immobilizing the spinal cord connected at its caudal end (category 1 patients) are sectioned or resected with better results than category 2 patients. (3) The growth rate of the spinal column in relation to that of the spinal cord influences the spinal cord tension. (4) Forcible cord stretching caused by flexion-extension exercises, a violent impact to the spinal column, or repeated jolting of the spine aggravate signs and symptoms. (5) Impaired oxidative metabolism associated with TCS is accentuated by any further imposition of hypoxemia, ischemia, or venous stasis, such as that which might be produced during surgical procedures. Fig. 21.1 Patchy distribution of sensory deficit (mostly to pain and temperature) is shown in the lower left limb. The dotted line indicates the analgesic area and the dashed lines the hypalgesic area. The grading system of Hoffman et al4 for patients with lipomyelomeningoceles (LMMCs) (Table 21.1) according to the signs and symptoms is valuable to determine the patient’s prognosis (Chapters 3 and 11). This system indicates that the more severe the signs and symptoms, the worse is the outcome. In mild forms of TCS described as type 1 and type 2 (Chapter 3), signs and symptoms often fluctuate. This fluctuation is noted in both adult and young patients. It is not uncommon that after a few days to a few weeks of resting, the patients are often relieved of back and leg pain, and improved in motor, sensory (Fig. 21.1), and bladder function. The surgical prognosis of these patients is excellent (Chapter 11). Conservative treatment in these patients is discussed later in this chapter. Table 21.1 Grading System for Lipomyelomeningoceles
Severity of Signs and Symptoms and Prognosis
| Grade | Explanation |
| 0 | No significant neurological, orthopedic, or urological problem; the patient may have reflex changes and/or sensory deficits |
| 1 | Minimal weakness and/or muscle-wasting and/or foot deformity affecting only one leg without significant gait disturbance; normal bladder and sphincter function |
| 2 | Neurogenic bladder alone or combined with minimal weakness of one leg, or intact bladder function with minimal weakness affecting both legs |
| 3 | Moderate to severe weakness of one leg producing gait disturbance with or without neurogenic bladder, or minimal weakness of both legs combined with neurogenic bladder |
| 4 | Severe paraparesis requiring aids for walking, with or without neurogenic bladder |
| 4 | Inability to ambulate |
Source: Hoffman HJ, Taecholarn C, Hendrick EB, et al. Management of lipomyelomeningoceles: experience at the Hospital for Sick Children, Toronto. J Neurosurg 1985;62:1–8. Reprinted with permission.
Inelastic Filum
The elasticity of the filum determines the degree of cord tethering (Chapters 4, 7, 15, and 20). Fat tissue is soft and elastic but becomes the source of excessive cord tension when the fibrous component increases in fibroadipose tissue. The vulnerability of the spinal cord seems dependent on the volume of each cord segment.5 The elongated spinal cord (from L1 to S1 segments) is thinner than the normal cord and therefore vulnerable to stretching, whereas the diameter of the conus may be greater than that seen in the normal individual (Chapter 18).
Spinal Cord versus Vertebral Column Growth
Although traction effect of an inelastic filum on the lumbosacral cord determines the occurrence of TCS, the growth rate of the vertebral column also influences the severity of the increased tension within the spinal cord that is tethered at its caudal end. From 8 to 9 weeks of gestation, the normal spinal cord begins to ascend in the spinal canal as the growth of the vertebral column is accelerated. The caudal end of the spinal cord is opposite the L3 vertebra by the time of birth5,6 (Chapters 2 and 15), and it ascends farther to the T12, L1, or L2 vertebral level by 3 years of age6,7 (Chapters 2 and 10). Because no spinal cord ascension has been recorded thereafter, the overall growth rate of the spinal cord is assumed to be equal to that of the vertebral column after this age (Fig. 21.2).8 A certain degree of tension exists within the normal cord.2,9 If the spinal cord is anchored by an inelastic filum at or below L3 at the age of 4 years or older, the growth rate of the lumbosacral cord may not match that of the vertebral column10,11 and is likely to be under excessive tension and further develop neuronal dysfunction. Both scoliosis and accentuated lordosis allow the spinal cord to take the shortest course in the spinal canal.12,13 These curvature changes serve as an appropriate means to minimize the tension within the spinal cord. The incidence of a normal (L2–3 interspace or higher) or slightly lower (L3 vertebral level) location of the caudal end of the spinal cord is much higher in adults with TCS (Chapter 15) than in children with TCS14,15 (Chapter 9) (Fig. 21.3). These data can explain the delay of TCS manifestation in adulthood.
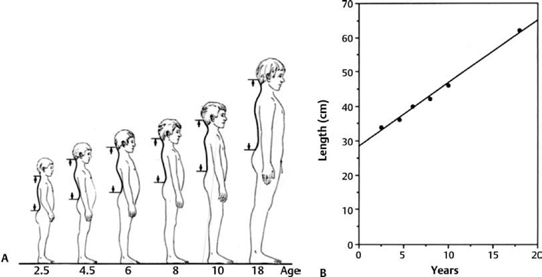
Fig. 21.2 (A) Diagram shows the growth of the spinal column. (B) The growth rate of the spinal column was calculated by measuring the distance between the C1 and S2 vertebrae in humans ranging from 2.5 to 18 years of age.
Forcible Stretching of the Spinal Cord by Spinal Movement
Performance of flexion-extension exercises or any other strenuous activities associated with spine curvature changes can strain the spinal cord, causing progression of TCS. The fact that the pressure on a large lipoma or LMMC transmits a stretching force to the spinal cord should alert the patients of neurological catastrophe resulting from undue pressure on these anomalies (Chapter 11).
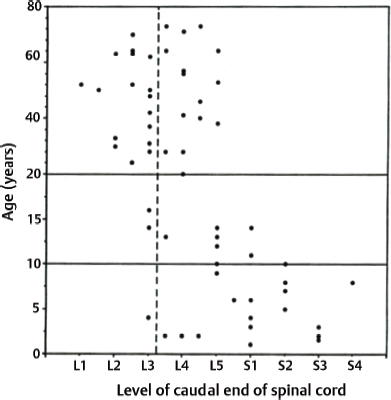
Fig. 21.3 Graph showing the location of the caudal end of the spinal cord relative to the vertebral level. The location of the caudal end is crowded at the L5-S2 vertebral level in TCS patients younger than 20 years of age, and at the L2–4 levels in those 20 years or older.
Oxidative Metabolism
Intraoperative redox studies of cytochrome a,a3 have indicated that the more functionally impaired the spinal cord function, the more severe are the derangements in oxidative metabolism. This apparent link between neurological and metabolic dysfunction occurred regardless of whether the TCS symptomatology started spontaneously or was initiated by a back injury. Type 1 patients had the best prospects for regaining normal spinal cord function after untethering procedures (Chapter 3). Although the sequence of neurological improvement was slower than in type 1 patients, type 2 patients regained normal or nearly normal motor, sensory, and bladder function. In type 3 patients, recovery from oxidative metabolic impairment was incomplete even though the spinal cord was relaxed after untethering.
Type 1 patients correspond to grades 0 and 1 of the grading system of Hoffman et al.4 Type 2 patients correspond to grades 2 and 3, and type 3 patients to grade 4 and partly grade 3. These correlations allow for the following conclusions. Type 2 patients should be operated on as soon as the diagnosis is made. The type 1 and type 3 patients need careful evaluation, because neurological signs and symptoms fluctuate; for example, type 1 patients may become almost asymptomatic after 2 weeks of rest, and subtle progression in type 3 patients may be overlooked or undetected. Any degree of symptomatic worsening is an indication for surgical untethering.
Treatment
Conservative Treatment
Conservative treatment is desirable for patients with no neurological and musculoskeletal abnormalities despite MRI evidence of an elongated spinal cord attached to a thick filum or a lipoma. Patients with back and leg pain that is accentuated by typical postural changes but with minimal deficit (Chapters 3 and 15) may show only subtle signs of TCS. Surgical untethering protects those patients from progression of neurological deficit and musculoskeletal deformities.
Conservative treatment mainly consists of preventing spinal cord stretching that occurs as a result of straightening the lumbosacral lordosis and consequent elongation of the spinal canal. Patients must be instructed to avoid such postures as (1) sitting legs crossed in a Buddha pose or Yoga sitting, (2) bending over the sink or lavatory16,17 (see Fig. 15.3 in chapter 15), (3) holding the baby or heavier object at the waist level, (4) holding any weight while standing that causes back and leg pain, (5) sleeping supine in the bed overnight, (6) for woman to lie supine during intercourse, (7) sitting in a slouching position, (8) driving or riding in the car on a bumpy road, until back pain is aggravated, (9) walking, running, or horseback riding long enough to cause back and leg discomfort. (10) deep bending such as athletic practice, toe touching (Fig. 21.4), and high leg kicking, although these exercises mainly use flexion of the hip joints and thoracic spine. These exercises could also straighten the lumbosacral spine and should be avoided. Those patients who show Lhermitte sign should be instructed to avoid hyperflexion of the head and neck.
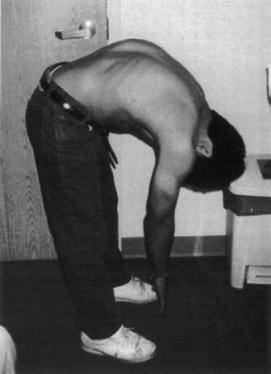
Fig. 21.4 This 16-year-old boy with severe rotational scoliosis began to complain of back and leg pain for several months, interfering with soccer skills. Repeated toe-touch exercises aggravated the pain. Within a few weeks after untethering surgery, he was free of back and leg pain. In 6 months, he underwent corrective spinal fixation surgery and returned to playing soccer.
Because back and leg pain is muscular in nature, muscle relaxants are effective, including (1) medications that control overexcited interneurons in the spinal cord, such as phenobarbital (not as a tranquilizer) and carisoprodol (Soma); (2) muscle relaxants such as valium, methocarbamol (Robaxin); (3) nonsteroidal antiinflammatory agents, such as ibuprofen, celecoxib, (4) steroid for limited period of severe symptomatic exacerbation, (5) opioid derivatives that may be prescribed for only severe pain that occurs after strenuous physical activities with only intermittent usage, but not longer than a few months to avoid dependency or addiction.
TCS patients should be warned of worsening signs and symptoms by any Valsalva-type maneuver that causes venous congestion and resultant ischemia,18 and any possibility of tissue hypoxia such as might be produced by strenuous activity, such as skiing at high altitude or diving for a prolonged time. Lying supine on a lipomatous mass during intercourse can result in paraplegia (Hoffman HJ, personal communication,1995).
Surgical Treatment for TCS due to an Inelastic Filum, Sacral MMC, or Caudal MMC
TCS is a syndrome characterized by neurological dysfunction secondary to high tension within the spinal cord, rather than to histological neuronal damage. Intraoperative noninvasive redox studies of cytochrome a,a3 have demonstrated that a link exists between impairment of oxidative metabolism, regulated by mitochondria in the tethered lumbosacral spinal cord, and neurological dysfunction. Prognosis after surgery appears to be definable by how large the shift toward oxidation of the mitochondrial cytochromes occurs after untethering. Therefore, spinal cord function should return to normal if untethering procedures are performed, as long as the neurons are still metabolically and electrophysiologically at a functional level (Chapters 3, 11, and 15). These scientific results indicate that an untethering procedure should be done as soon as even mild signs and symptoms are noted.
Surgical Treatment for Early Tethered Cord Syndrome
Case 1
The following case is an example of TCS manifestation after repeated strenuous exercises and perfect surgical outcome when treatment was provided in the early stages of TCS. This 22-year-old paramedic trainee presented with difficulty in voiding and intermittent left groin and testicular pain mixed with paresthesia (pins and needles) since 14 years of age. He was toilet trained and walked at 2 years of age. His mother knew that his feet were flat early in childhood, but she, as well as the patient, had noticed an increasingly high arch of both feet and curling of toes (hammertoes) for several years. He recalled that he was not athletic as a child because his body was stiff. The patient underwent intensive physical training and gained flexibility to sit in a yoga position and to touch his toes by his late teens.
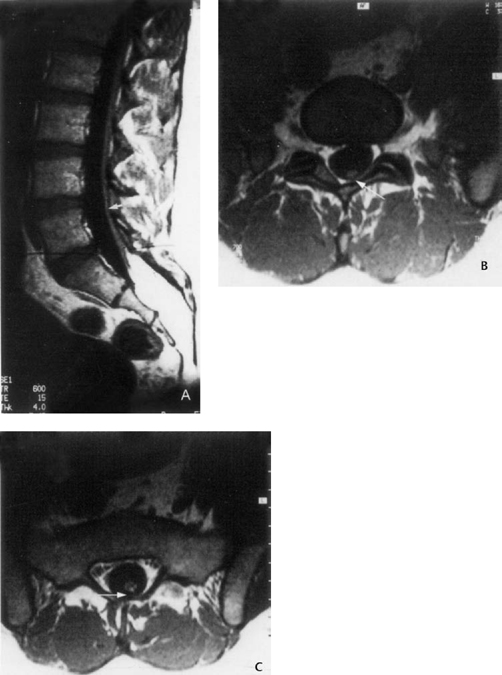
On examination, this patient exhibited a slight inversion of the left foot on tiptoe walking (indicating peroneus longus weakness), minimum hypalgesia in the dorsum of both feet, and coccygeal subcutaneous fat and tufts of hair. Ancillary MRI studies showed that there was adipose tissue in the low sacral and coccygeal vertebral canal. Also, testing demonstrated a neurogenic bladder with motor instability of the tonic type and a residual urine volume of 150 mL (via cystometrogram). MRI showed a sacral intraspinal lipoma and posteriorly displaced filum, which was touching the posterior arachnoid membrane (Fig. 21.5).
At operation, an inelastic filum was found continuous to a lower sacral and coccygeal fat mass. After cord untethering by sectioning the filum, the patient was relieved of the testicular pain immediately and of incontinence within 1 week. Within 2 months, he noticed that the high arching of the feet and the hammertoes were decreasing. He returned to normal training within 6 weeks.
Case 2
This case represents TCS that developed late in life. This 72-year-old man complained of pain in the back and legs, and weakness and numbness in his distal leg muscles for 9 months. Weakness of the dorsiflexors and plantar flexors of the feet and toes and of the foot everter was found. At operation, the caudal end of the spinal cord was opposite the L5 vertebral level continuous to the fibroadipose filum. The conus ascended 1 cm after sectioning the filum (Fig. 21.6). The patient was relived of pain and improved in motor function. Preoperative spinal cord dysfunction was attributed to the progression of osteoarthritic stenosis that caused restriction of the cord and filum movement and consequently increased cord tension.
Prophylactic Untethering Procedures
The benefits (if any) of prophylactic surgery for potential TCS in patients with cord elongation and filum thickening are still debatable.19–23 The majority of these patients develop symptomatology early in life, but an increasing number of patients24–26 develop TCS in adulthood (Chapter 15), as late as 80 years of age in our series. However, it is clear that most of the patients with an MMC or LMMC are anticipated to develop TCS, and its prevention would be repair of these anomalies and cord untethering in category 127 and category 2 if possible.
Surgical Treatment for Bladder Control
Chapters 7, 8 and 19 are dedicated to neurourological function in Chapter 13 to sphincter control.
The importance of detecting bladder and rectal control in patients with TCS in its early stage has been recognized. Bladder dysfunction as an early symptom of TCS is reversible but not in the later stage. For this reason, it is imperative for parents to watch diapers of their infant with sacral MMC or LMMC every 15 minutes for 2 hours periodically four times a day. When periodical wetting changes to constant wetting, urgent surgical untethering is mandatory to prevent rapid deterioration of conus function. The following anatomical analysis may explain conus dysfunction: as observed in Fig. 21.7; nerve roots of one cord segment take a horizontal course, as do all the nerve roots at 8 to 9 weeks gestation. It is apparent that this cord segment moved neither cephalad nor caudad in relation to the vertebral column during any period of growth. Considering its nerve roots traveling caudad to their dural exits, the cord segment above the horizontal roots is likely to have stretched or grown cephalad. Likewise, the cord segment below the horizontal roots could have grown caudad, along with the caudad growth of the sacral vertebrae (Fig. 21.8). It is postulated that the segments above and below the horizontal nerve roots are under excessive tension as the cause of TCS in this patient.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







