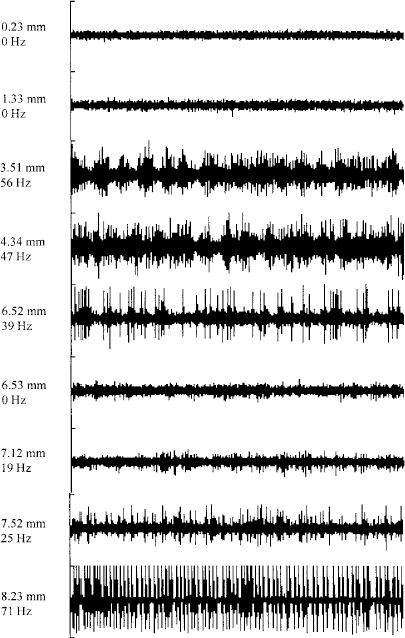
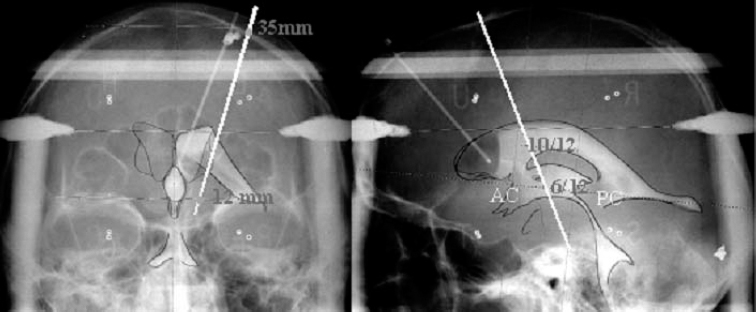
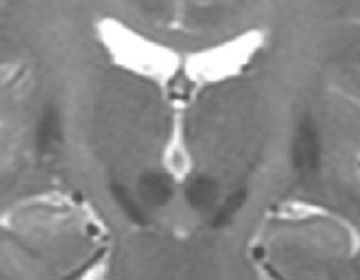
19 The subthalamic nucleus is a small structure of the basal ganglia characterized by a high neuronal density.1,2 It has been shown to play an important role in the control of movement. The nucleus is linked with hemiballismus because vascular accidents in the region including the subthalamic nucleus were often associated with the appearance of abnormal involuntary movements.3,4 Recently several studies have demonstrated that the subthalamic nucleus is implicated in the pathophysiology of Parkinson’s disease. Lesions in that region have been shown to induce an improvement in motor symptoms in monkeys rendered parkinsonian by systemic injections of 1-methyl-4-phenyl-1,2,3,6-tetrahydropine (MPTP).5,6 Because the beneficial effect of such lesions is accompanied by the appearance of abnormal movements, it was difficult to propose lesional surgery for the treatment of Parkinson’s disease. To avoid the side effects, we have replaced ablative lesions with high-frequency stimulation (HFS), which has been shown to reverse the three cardinal motor symptoms—akinesia, rigidity, and tremor—in primate models of parkinsonism7–9 and in patients suffering from severe parkinsonism.10–13 The technique of deep brain stimulation for the treatment of Parkinson’s disease has evolved very rapidly since its introduction in 1987,14 and the subthalamic nucleus has become the preferred target in the past few years because HFS in this nucleus improves all cardinal symptoms, including resting tremor. Such a large degree of benefit in parkinsonian features relies on two crucial parameters that must be taken into consideration: proper selection of patients and accuracy in targeting the subthalamic nucleus. To ensure a precise localization of the ideal target, the stereotactic implantation of the stimulating electrodes is performed with the patient under local anesthesia and after a 12-hour withdrawal of antiparkinsonian drugs. Because of individual variations, the final target could be different from the theoretical target and needs to be defined by the following complementary data: ventriculography, magnetic resonance imaging, intraoperative microrecording, and intraoperative stimulation. Geometric landmarks obtained from ventriculography (Fig. 19–1) using Guiot’s scheme based on the AC–PC line.15–17 After ventricular puncture, injection of 6.5 mL of Iopamiron 200 (Schering) is performed. Anteroposterior and lateral x-rays are taken in the supine and ventral position, to visualize correctly the PC and AC. The radiological images that are obtained provide a very precise delineation of the midline of the third ventricle and of the AC and posterior PC, which are used as a coordinate system basis to calculate a statistical estimation of the coordinates of the targets and to build the Guiot’s scheme,18 which will be used as the template for final targeting. These coordinates were initially derived from the stereotactic atlases of Schaltenbrand and Wahren and of Talairach,19,20 then further validated by the correlation with the clinical results. The average dimensions of the third ventricle (V3) are AC–PC length 24.63 ± 1.64 mm (N = 153, extrema: 21.21–27.59), height of thalamus (HT) 16.62 ± 1.47 mm (N = 153, extrema: 12.57–19.95), width of V3 5.22 ± 1.64 mm (N = 153, extrema: 1.52–19.46). On the sagittal view, the subthalamic nucleus is situated in the middle third of the AC–PC distance, 0 to 6 mm below this plane, and on the anteroposterior view, at 10 to 14 mm from the midline (coordinates: anteroposterior: 5.28 ± 0.58 1/12 degree of AC–PC length, extrema: 2.88–7.08; vertical: −1.22 ± 0.65 1/8 degree of HT, extrema: −3.29 ± 0.19 degree; laterality 12.14 ± 2.05 mm from midline, extrema 9−15.2). FIGURE 19–1 Determination of the subthalamic DBS target based on the third ventricle anatomical landmarks. The subthalamic target extends over the middle third of the AC–PC, below the line. On the anteroposterior view, the average laterality is 12 mm, and the subthalamic nucleus extends from 11 to 13 mm. The entry point is around 35 mm and will be adjusted on the MRI planning to avoid the F1-F2 sulcus. On the lateral view, the trajectory is a theoretical angle on the AC–PC that is 63.57 degrees + 2.34 on the basis of the Guiot scheme. Currently, stereotactic MRI is the key investigation for localization in the subthalamic nucleus. T1-weighted sagittal sections and T2-weighted coronal sections (Fig. 19–2) provide the coordinates of the small almond-shaped subthalamic nucleus, 1 to 2 mm anterior to the red nucleus, 2 to 3 mm superior and slightly lateral to the substantia nigra, externally limited by the internal capsule and posterior to the mamillary bodies15,17 allowing precise determination of the coordinates of the center of this small nucleus. However, these data must be carefully checked, as MRIs may be distorted for several reasons: inhomogeneity of the primary magnetic field due to improper tuning of the shim gradients or due to changes of the magnetic susceptibility from the patient himself, even though the patient may not have metallic (e.g., dental) implants. As a result, the spatial data that are dependent on a frequency coding of the space by linear magnetic gradient may prove erroneous, resulting in the wrong position of the corresponding point on the final MRIs. These errors, moreover, depend on the direction in which one looks at the values (coaxial or orthogonal to the main magnetic field) as well as on the sequences of the MR picture acquisition protocol. They are larger at distances from the axis of the main magnet, and therefore are smaller for the subthalamic nucleus than for the Vim or the GPi. They can be minimized by a careful tuning of the machines. Fusion of the MRIs with CT data in theory may circumvent these errors, provided that the fusion process reformat the MR data in the nondeformed space represented by the CT image. Currently available industrial software does not achieve this reformatting, but provides only the best match of the two sets of data. FIGURE 19–2 MRIs of the subthalamic target on coronal T2-weighted sections. The pallidum, subthalamic nucleus, and substantia nigra, as well as the red nucleus, are visible as strong hyposignals. The thalamic complex can be seen as outlined by the oblique relative hypersignal of the internal capsule. The subthalamic nucleus represents the upper half of the subthalamic nucleus–substantia nigra complex, outlined laterally by the internal capsule. Intraoperative electrophysiology, using extracellular single-unit microrecording, helps to identify with precision the limits of the subthalamic nucleus and the sensorimotor region of this nucleus. For electrophysiological microrecordings, as well as for intraoperative stimulation, we use a microdrive that allows five simultaneous parallel trajectories, with four trajectories each 2 mm apart from a central one. Tungsten bipolar microelectrodes (FHC, Bowdoinham, USA) with an impedance between 2 and 6 MOhms (measured at 1000 Hz) were introduced into each trajectory for single-unit recordings. Neuronal activity was recorded at various sites along the trajectory using the NeuroTrek system (AlphaOmega, Nazareth, Israel), equipped with five isolated preamplifiers to record five simultaneous channels. The acquisition program contains a spike discriminator, which allows the recording of only single-unit neuronal activity. The relation of neuronal activity in the subthalamic nucleus to passive manipulation of different body parts was studied. The study consisted of the examination of passive joint movements, muscle palpation, active movements of the jaws and tongue, and orofacial light touch. The presence or the absence of neuronal responses was determined by listening to the recorded cellular activity using an audio amplification. Single-unit recordings are started at the AC–PC line level and stopped when the cellular activity is characteristic of the substantia nigra pars reticulata. Figure 19–3 shows a representative example of the trajectories for targeting the subthalamic nucleus. During a typical exploratory track, the first recorded structure is the lower part of the thalamus in which the majority of cells exhibit a burst discharge pattern. The background noise then decreases in the zona incerta. Penetration of the electrode tip into the subthalamic nucleus is characterized by a sudden increase in the level of background noise, reflecting an increased density of active neuronal cell bodies. Spontaneously active neurons are recorded as single-cell activity, typically with spikes at the same amplitude, or as multiunit activity with two or three cells characterized by different amplitudes of the spikes. The firing rate of spontaneously active cells in the subthalamic nucleus is around 30 to 40 spikes/sec.21–24 Most of the time, multiunit activity is easily observed, and fine-tuning of the microdrive allows single-unit recordings. Two main patterns of neuronal discharges in the subthalamic nucleus were observed: tonic irregular active neurons and bursty neurons. In the second population of cells, periodic oscillatory bursts synchronous to the rest tremor were observed in patients with parkinsonian rest tremor.21–24 Thirty to 40% of recorded neurons in the subthalamic nucleus responded to passive and/or active movement of the contralateral part of the body, and only a few cells responded to the ipsilateral limbs (~5%). Most of the tremor-related cells are responsive to passive movements. The distribution of all the cells with movement-related activity is in the dorsal two thirds of the nucleus corresponding to the sensorimotor region of the subthalamic nucleus.
Correlation between Microelectrode Recording and Clinical Effects of High-Frequency Stimulation of the Subthalamic Nucleus
ABDELHAMID BENAZZOUZ, ADNAN KOUDSIE, PIERRE POLLAK, AND ALIM-LOUIS BENABID
Ventriculography
Stereotactic Magnetic Resonance Imaging
Intraoperative Microrecording
Neupsy Key
Fastest Neupsy Insight Engine