Fig. 23.1
CT scans showing defects in anterior skull base with encephalocoeles, arrows pointing towards the bony defects (Courtesy Dr. S. Mathur, Royal Preston Hospital)
To rule out high intracranial pressure or malignant lesions as a causative factor. If there is tumour or raised intracranial pressure, then these have to be appropriately managed before considering any surgical repair.
To undertake necessary corrective measures. The choice will usually be between surgical intervention and a conservative approach, insofar as some leaks will repair themselves. Some post-traumatic CSF leaks may spontaneously settle. The decision concerning which approach to adopt needs to be made on a case-by-case basis.
Spontaneous CSF leaks can be challenging to manage owing to frequent recurrences. Furthermore, individuals with this disorder seem also to rise from a distinct demographic group. Spontaneous CSF leaks account for 3–4 % of cases of CSF rhinorrhoea, and recurrences of spontaneous CSF leaks are seen mainly in patients who have raised intracranial pressure. In one study, patients with a spontaneous CSF leak and raised ICP had a 46 % recurrence rate (Mirza et al. 2005). Paradoxically, the presence of a CSF leak may keep patients with raised ICP symptom-free; classic signs may only develop after the leak is repaired (Pérez et al. 2013). Most spontaneous CSF leaks will self-repair within 7–10 days, and this may be helped by oral acetazolamide, an inhibitor of carbonic anhydrase, which is now the standard treatment for benign intracranial hypertension (Chaaban et al. 2013).
In tracing the source of a leak, the intrathecal contrast-enhanced MR cisternography (CEMRC), which can identify 90 % of surgically proven cases, is more sensitive than T2-weighted MR cisternography (T2MRC), identifying 65 % of cases (Ecin et al. 2013). The overall, detection sensitivity, specificity, positive predictive values and negative predictive values are typically 92, 80, 76 and 93 % for CEMRC and 56, 77, 64 and 71 % for T2MRC, respectively (Aydin et al. 2008; Reiche et al. 2012). High-resolution CT was accurate in 93 % of patients, whilst MR cisternography was accurate in 89 % (Shetty et al. 1998).
Although relatively safe, the use of intrathecal fluorescein injection is not without risk (Keerl et al. 2004; Jacob et al. 2008). As an alternative to this invasive approach, endoscopic endonasal topical application of fluorescein has been suggested (Saafan et al. 2006). This relies on either detecting a colour change in the fluorescence triggered by the pH of CSF or simply observing the washing away of the fluorescein solution by flowing CSF. Such an approach was reported to accurately identifying the presence of CSF fistulae, though not always possible to identify the accurate site of the leak. It has the advantage that it is a quick and simple outpatient clinic test, although further studies are required.
When CSF rhinorrhoea is identified postoperatively, there is a role for conservative management with lumbar drain, bed rest with head elevation and stool softeners up to 4–6 weeks. Persistent rhinorrhoea, however, will need surgical management due to the risk of ascending meningitis and consequent catastrophic sequelae. Generally, all cases of spontaneous CSF leak, intermittent CSF leak, delayed post-traumatic leak, CSF leak with history of meningitis and CSF otorrhoea presenting as rhinorrhoea require surgical closure. Open craniotomy surgery was prevalent prior to the advent of endoscopic surgery, but endoscopic intranasal management of CSF leaks is now preferred, as this approach is generally associated with high success rates and less morbidity. The key is to place a graft between the skull base bone and dura mater across the CSF leak site (Fig. 23.2). The graft material can be free tissue: fascia lata, abdominal fat, septal or turbinate mucosa or composite grafts with perichondrium or periosteum and mucosa. Multiple techniques have been described and include endonasal repair using septal flap or osteomucoperiosteal flaps through an external ethmoidectomy approach or endoscopic intranasal approach. After the graft is placed, the graft is supported with use of fibrin glue, Gelfoam pieces/Surgicel or other absorbable nasal packs first. The rest of nasal cavity can be packed with rapid Rhino pack/Merocel/BIPP according to surgeon preference. Patient should be advised bed rest with head end elevation of 45° and stool softeners for at least 48 h. Although endoscopic closure is now the treatment of choice (Martin and Loehrl 2007), there remains a recurrence rate of ~10 %.
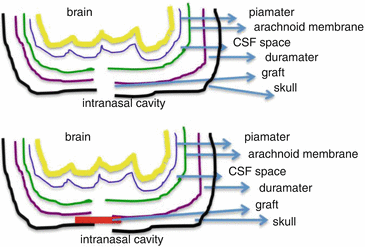

Fig. 23.2
Schematic diagram for CSF leak and placement of graft for repair of defect
Whilst trauma-related CSF fistulas can also resolve without intervention, these cases carry a greater risk of an ascending infection, and surgical closure of leaks and defects should be considered. Although the value of prophylactic antibiotics in CSF leaks is debatable (Brodie 1997), this precaution likely justified until the remaining questions are answered. Contemporary surgical innovations towards repair include the use of fibrin glue (Yang et al. 2013).
23.2.3 Transsphenoidal Pituitary Surgery and Transmastoid Schwannoma Surgery
CSF leak can occur after pituitary surgery through the sphenoid sinus. Transsphenoidal surgery has been the gold standard for intra- and suprasellar lesions for nearly half a century. Following this approach, CSF fistulas occur in ~2 % of cases (Malik et al. 2012). Intra-operative CSF leakage can occur in ~20 % of cases (Nishioka et al. 2005). In such cases after covering the defect with graft, the sphenoid sinus is obliterated with abdominal fat (Zie and Jimenez 2013). Similarly following vestibular schwannoma surgery, it is important to have watertight dural closure, typically involving the use of fascia lata over the dural closure, obliteration of the mastoid cavity with abdominal fat, occlusion of all air cell tracts with bone wax and use of palva periosteal flap to reduce the risk of CSF leak.
23.2.4 Spinal Surgery
CSF leaks are relatively common following spinal surgery, and surgeons can be confronted with a draining operative wound with or without deep lying fluid accumulation. Most frequently, the differential diagnoses include seroma, infection and CSF leakage. Imaging can show fluid accumulation but does not necessarily differentiate between diagnoses. Duro-pleural fistula will result in a chronic transudative pleural effusion with a watery fluid having a low total protein (Huggins and Sahn 2003) that can prove problematic to distinguish from a true CSF leak. Testing for asialotransferrin can be an effective means of identifying CSF leakage in such cases (Nyunoya et al. 2003; Haft et al. 2004).
Whilst postspinal surgery midline dural tears are easy to repair, ventral tears are more problematic. As with transsphenoidal surgery, spinal surgery approaches to sealing a leak involve using a sheet of fat from a patient’s subcutaneous tissue to cover the dural suture site, exposed dura, followed by application of fibrin glue and surgical/Gelfoam (Black 2002).
Post-lumbar puncture (LP) headache is probably the most common consequence of spinal intervention and is caused by a persistent CSF leak. One common approach to minimising the risk of this is to use the patient’s own blood to form a clot over the puncture site extradurally (epidural blood patch).
23.2.5 Shunt CSF-orrhoea
Extreme examples of CSF-orrhoea are seen in abdominal CSF pseudocysts, infrequent but well recognised complications of ventriculoperitoneal shunts (VPS). In CSF pseudocysts, litres of fluid can accumulate, and presenting symptoms and signs are mainly related to abdominal complaints (Ersahin et al. 1996). The mechanisms by which CSF pseudocysts form are not clear. They may take years to develop, and it is often the case that the patient has not undergone any prior abdominal surgery (other than shunt placement). Manifest shunt malfunction is not a prominent feature. Whilst antecedent shunt infections associate only rarely, CSF obtained at the time of surgery is infected in about one third of cases (Hahn et al. 1985–1986). Suggested surgical management consists of either a contralateral VPS or placement of a ventriculoatrial shunt (Hahn et al. vide supra). Diagnosis is usually by abdominal ultrasound and/or CT scan. An intra-abdominal inflammatory process is widely accepted as a hypothesis for the formation of a pseudocyst. In a meta-review the rate of mechanical malfunction ranged from 8 to 64 %, whilst the rate of infection ranged from 3 to 12 % of shunt operations (Wong et al. 2012). Non-cystic variations include CSF ascites (Dean and Keller 1972)
23.3 Confirmation of CSF
The identification of an unknown fluid as being, or containing, CSF clearly depends upon identifying a components or components that characterise CSF. In times past this involved measuring chloride (Cl-), glucose and total protein. It is now widely realised that these tests do not offer the necessary discriminatory efficiency (Chan et al. 2004) and this analytical approach is no longer tenable (Mantur et al. 2011), even though there have been brave efforts to maintain their use (Baker et al. 2005).
Contemporary methods approach the problem by measuring molecular biomarkers, typically CSF-enriched proteins (Tabaouti et al. 2009).
23.3.1 Biomarkers of CSF
23.3.1.1 Asialotransferrin
Transferrin is protein used to transport iron throughout the body. All body fluids contain transferrin. Transferrin is also a glycoprotein, which means that it has oligosaccharide carbohydrate (glycan) chains incorporated into its structure. These glycan chains terminate in sialic acid (N-acetylneuraminic acid) moieties. One significant feature of sialic acid is that it carries a negative charge. This means that sialic acid contributes to the overall charge on the transferrin molecule. Electrophoresis is a method that is used to separate protein molecules on the basis of their charge. The most common form of transferrin in blood serum has 4 sialic acid residues. Asialotransferrin is a rare form of transferrin that has no sialic acid residues. Asialotransferrin, however, is present in significant amounts in perilymph, aqueous humour and CSF. Significantly, it is not present in either tears or rhinitis fluid. Using a combination of electrophoresis and immunoblotting, it is possible to separate and identify glycoforms of transferrin that can be used to distinguish CSF from other serum-based fluids (Fig. 23.3). CSF is characterised by the presence of a significant proportion of asialotransferrin. Approximately 30 % of CSF transferrin is in the form of asialotransferrin. Asialotransferrin is also referred to as tau-transferrin and β 2 -transferrin, although the use of asialotransferrin is preferred, as this is less ambiguous.
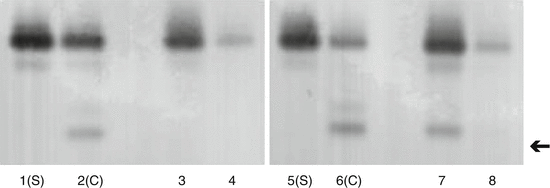

Fig. 23.3
Immunoblotting for transferrin glycoforms in rhinorrhoea fluids. Lanes 1, 2, 5 and 6 are control serum (S) and CSF (C). Lanes 3, 4 and 8 are rhinorrhoea fluids negative for CSF. Lane 7 is a sample of CSF rhinorrhoea. The asialotransferrin band in indicated by the arrow
Identifying an unknown fluid as containing CSF simply requires performing electrophoresis and immunoblotting for transferrin (Keir et al. 1992). The presence of the asialotransferrin band signifies the presence of CSF. The immunoblotting method is sufficiently sensitive to identify CSF even when contaminated with either tears or nasal secretion. Indeed, CSF can be detected when it comprises 10 % or more of an admixture with other non-CSF fluids. Although technically straightforward, the main limitation to the asialotransferrin test is the time taken to perform the electrophoresis and immunoblotting.
In cases of CSF rhinorrhoea and skull base fistulas, asialotransferrin has a detection sensitivity of 94–100 % and specificity of 98–100 % (Marshall et al. 1999). Of course, asialotransferrin testing can also be useful to exclude rhinorrhoea feigning as CSF leak (Bateman and Jones 2000). The test has also been successful in identifying cases of CSF pleural effusion following placement of a ventriculoperitoneal shunt (Smith and Cohen 2009).
Asialotransferrin is found in perilymphatic fluid produced by the cochlea. A perilymph fistula is defined as a leak of perilymph at the oval or round window and is most commonly caused by either barometric trauma associated with either flying or diving, ear surgery or, rarely, head trauma. The very existence and definition of perilymph fistulas remains a contentious area that is fraught with difficulties (Hornibrook 2012). Not least of the problems is that the volume of perilymph is only some 75 μl in toto, so collecting and analysing the fluid is difficult; a typical clinical sample is typically less than 10 μl and is often contaminated with other fluids. This makes the laboratory analysis challenging. Furthermore, the concentration of asialotransferrin in perilymph is only half that of CSF (Thalmann et al. 1994). Irrespective of these technical barriers, asialotransferrin has been successfully applied to the detection of perilymphatic fluid leaks (Skedros et al. 1993). Future developments, such as detection of cochlin-tomoprotein (CTP) may yet prove to be a more specific marker for this fluid (Ikezeno et al. 2010).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






