CHAPTER 13 CT Evaluation of Meningiomas
INTRODUCTION
The majority of meningiomas have a typical appearance on CT, and the diagnosis is straightforward in these cases.1 The nonenhanced CT examination detects 85% and contrast-enhanced CT detects 95% of all intracranial meningiomas.2,3 Small intraventricular meningiomas or small lesions without surrounding brain edema may be missed on nonenhanced CT examinations. The typical meningioma appears as a sharply delineated round or hemispherical mass of iso- to high density on nonenhanced CT.
CT TECHNOLOGY
CT is one of the most outstanding inventions in the history of medicine. CT has contributed to the development of all disciplines in medicine and revolutionized brain imaging by displaying the brain directly in two dimensions, hence contributing significantly to progress in neurologic sciences. The first CT was designed and built by the English engineer Sir Godfrey Newbold Hounsfield at EMI Research Laboratories in England. The first CT image was acquired at the Atkinson Morley Hospital in London in 1972 and displayed a frontal brain tumor, which immediately convinced the medical profession of this new technology’s diagnostic capability.4 Allan McLeod Cormack at Tufts University independently invented a mathematical technique for the CT scan. G.N. Hounsfield and A.M. Cormack shared the Nobel prize in Medicine in 1979 for their achievements.4 Since the production of the first CT equipment, which was called the EMI head scanner, major technical advances were made in faster patient scanning and faster image reconstruction. First-generation CT scanners acquired a single brain slice in about 5 minutes.5 The introduction of new technologies such as spiral and helical CT has significantly shortened the scanning time. Finally, multidetector row CT technology was introduced in 1998. With this new technology, which uses an array of detectors to scan up to 64 slices per gantry rotation, a tissue block of 250 mm scan range can be visualized in a mere 4 seconds, while a whole-body examination can be completed in about 30 seconds. Such short scan times are a major advantage in emergency, traumatic, pediatric, and neurovascular cases.
Although CT is already a very practical and versatile imaging modality, it is still undergoing rapid technologic improvement. With increasing scanning speed new clinical applications such as CT angiography, CT venography, and CT perfusion studies are possible. Lately, the quality of CT angiography was improved to such an extent that it rivals catheter angiography. Multidetector row CT reconstruction protocols such as multiplanar reformation (MPR), maximum intensity projection (MIP), and volume rendering techniques yield images in three dimensions with resolution equal to that of primary images. The high spatial and temporal resolution and the excellent quality of 3D reformations certainly are some of the factors that make CT the most widely used diagnostic modality at present. Dynamic CT angiography was introduced in 2005 by Matsumoto and colleagues6 to demonstrate the cerebral arteries and veins separately in arteriovenous malformations (AVM) and meningiomas. In 2007 this technique evolved into true dynamic imaging after the introduction of multidetector row CT.7 Multidetector row CTs with 64 detector rows perform 40 rotations around the patient in 2 seconds7 and provide hemodynamic information on local cerebral blood flow, demonstrate feeding arteries, nidi, and draining veins of cerebral AVMs within a scanning range of 32 mm. Dynamic CT examination of the whole brain will soon be possible with the use of 256 detector row CT scanners, which will make CT angiography the procedure of choice for evaluating every blood vessel in the body.7 Further refinements are also performed, such as bone-subtraction CT angiography, in which a nonenhanced scan is subtracted from the contrast-enhanced second scan to yield better resolution of vascular structures adjacent to bone.8
Advances are also being made to make CT a safer imaging modality. Radiation exposure related to CT is a major drawback. Although CT examinations comprise 15% of all radiologic examinations, CT is responsible for 70% of the total patient radiation dose, which introduces concerns on increased cancer risk, especially in children and young adults.9 Radiation dose-limiting paradigms such as “as low as reasonably allowable-ALARA” are more strictly implemented. Radiation dose delivered to the patient during a CT examination depends on scanning parameters such as x-ray tube current, tube rotation time, peak voltage, pitch, and x-ray collimation. Lowering any of these parameters results in decreased patient radiation dose, however, at the cost of increased noise and image quality. It is suggested that the ordering physician should primarily decide on the quality expected from the examination to yield satisfactory clinical information and guide the dose reduction. Similarly, dose modulation strategies are used to modify the x-ray tube current according to the patient’s thickness or attenuation, to match a preset reference image quality and noise level. The reference quality is determined according to the need, for example, reconstruction of thin slices is used during screening for small aneurysms, while thick scanning and thin reconstruction is used for evaluating the spine. Newer, multiplanar reconstruction techniques have eliminated the need for scanning in different planes, further decreasing patient radiation exposure. In addition, a few simple actions may also help further decrease the dose: repeat CT examinations for high-density lesions (such as hematomas) or for studies in which resolution is not a primary concern (e.g., perfusion CT) can be performed with low-dose settings. Unnecessary repeat examinations can be avoided, and lastly, scanning of the lens and the cornea can be avoided in repeat examinations.
CT IMAGING FEATURES OF MENINGIOMAS
Routine CT examination of an intracranial meningioma will assess the tumor location and extent; evaluate tumor margins; characterize the tumor; and determine the presence of any mass effect, edema, or brain herniations.10,11 A summary of common CT features is provided in Table 13-1. However, meningiomas are common tumors with variable appearances, so that specific consideration is warranted for each finding.
TABLE 13-1 Summary of CT findings in meningiomas.
| Criterion | Common findings |
|---|---|
| Location and extent | |
| Tumor shape | |
| Tumor margins | |
| Density | |
| Contrast enhancement | |
| Calcification | |
| Bone changes | |
| Edema | |
| Vasculature | Feeding or nearby vessels may be demonstrated |
Location and Extent
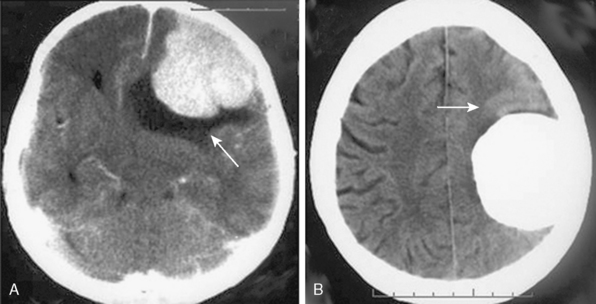
Meningiomas are extra-axial tumors, and the demonstration of this extra-axial origin is important for the diagnosis. In cases where the tumor attaches with a broad base to the dura or bone, the extra-axial origin is quite evident on CT examination. In certain cases, however, where the dural attachment is narrow it may not be easily demonstrated on CT.12 Two other CT findings that support the extra-axial localization are cerebrospinal fluid (CSF) clefts, surrounding or partially neighboring the tumor mass, and buckling of the cortex.12,13 CSF clefts appear as curvilinear hypodensities and form as a result of meningeal adhesions and tumor compression11 (Fig. 13-1A). This sign may not be evident on CT if the tumor has caused much mass effect. Buckling of the cortex, which denotes distortion and folding of the cortex and effacement of the sulcus under compression, is another finding common to extra-axial tumors14 (Fig. 13-1A, B).
Most common intracranial localizations for meningiomas, in decreasing order, are hemispheric convexity, parasagittal/falcine, sphenoid wing, olfactory groove, suprasellar and tuberculum sella, posterior fossa, cerebellar convexity, tentorium, cerebellopontine angle, foramen magnum, and intraventricular.10 The majority of these meningiomas are single. Multiple meningiomas may be encountered sporadically or as a manifestation of neurofibromatosis or meningiomatosis. Multiplicity is encountered in 10% of sporadic cases.15 Multiple meningiomas are encountered in 4.2% of neurofibromatosis type II patients.16–18 Multiplicity is common in families with a hereditary predisposition to meningiomas.18 Seldom, meningiomas can coexist with other intracranial tumors or even more rarely, intracranial meningiomas can be the site for metastasis of systemic cancer.
Tumor Shape and Margins
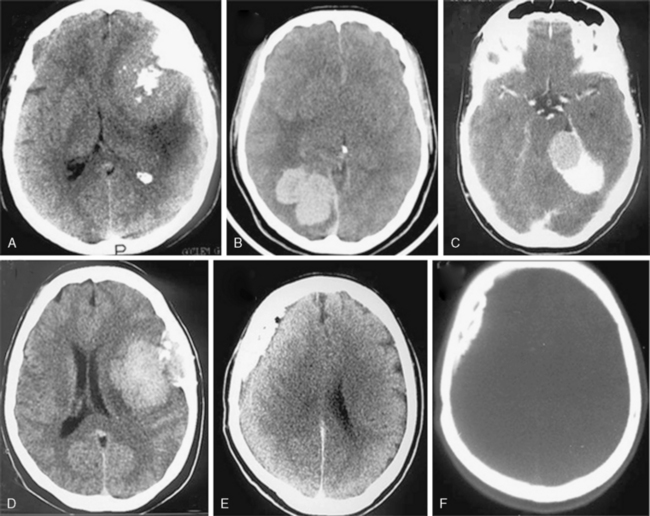
Most meningiomas are single, globoid (Fig. 13-2A) or lobulated tumors (Fig. 13-2B). The margins of the tumor are most commonly smooth and well circumscribed. Brain invasion may be seen in malignant meningiomas; however, most atypical and malignant meningiomas are sharply delineated from surrounding tissues.10,12 As described previously, CSF clefts may be seen around meningiomas.11 The shape may be influenced by surrounding anatomic structures (Fig. 13-2C). Multilobular meningiomas may also be encountered. “Mushrooming” is defined as the appearance of irregular, indistinct margins or finger-like extensions from the surface (Fig. 13-2D). Some authors have associated this finding with potentially malignant behavior.19 However, mushrooming may also be seen in a significant proportion of benign meningiomas. As demonstrated by this example, benign and malignant meningiomas can have very similar CT imaging findings. Malignant meningiomas may also have sharp, well demarcated margins and very little or no peritumoral edema. Therefore benign and malignant meningiomas cannot be differentiated based on CT findings alone.12
En plaque meningiomas, which are less common, grow flat along the dura like a carpet (Fig. 13-2E). A lucent line between the bone and the calcified en plaque meningioma corresponds to the dura (Fig. 13-2F) and is considered a specific sign for meningioma by some authors.20 En plaque meningiomas have a higher incidence of hyperostosis on CT.20
Density
On nonenhanced CT scans, 54% to 75% of meningiomas appear as homogeneous masses that are hyperdense compared to the adjacent brain cortex10,12,16 (Fig. 13-3A). Up to 25% appear isodense. Small isodense meningiomas may be overlooked on CT if they have no associated brain edema (Fig. 13-3B). Homogeneously hypodense meningiomas are quite uncommon and comprise 1% to 5% of all meningiomas.10,16 Hyperdensity can be attributed to hypervascularity, hypercellularity, reduced water content, or calcifications.2,16,21 Dense and widespread calcifications or acute hemorrhage will present as focal areas of marked hyperdensity.22 Gross hemorrhage within the tumor is rare in meningiomas. The density of acute hemorrhage is higher than that of the tumor but lower than that of calcifications (Fig. 13-3C). Follow-up CT scans will reveal decreasing density of the hemorrhage, which will finally become hypodense in 3 to 4 weeks. Large calcifications, cysts, necrosis, or hemorrhage can cause inhomogeneity of CT images. Such focal hypodense, nonenhancing areas, representing cystic changes, old hemorrhage, or necrosis, are encountered in 5% to 23% of all cases1,12 (Fig. 13-3D). Coexistence of hemorrhage and necrosis is extremely rare. Although extremely rare, metastasis into meningiomas from systemic cancer can also be seen.23 Lipoblastic or xanthomatous variants of meningiomas are extremely rare and may appear markedly hypodense on CT.24
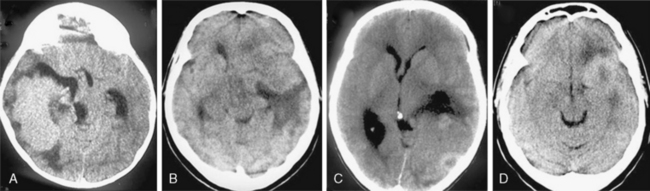
FIGURE 13-3 The typical meningioma appears hyperdense on nonenhanced CT images. The hyperdensity is easily appreciated if there is associated peritumoral edema (A). Small isodense meningiomas, on the other hand, may be missed in the absence of associated findings such as edema (B). Acute hemorrhage will appear denser than the parent mass (C) but less so than calcifications (see Fig. 13-4). Small areas of hypodensity in the meningioma may indicate cystic changes, old hemorrhage, or necrosis (D).
(Courtesy of Canan Erzan, MD, Marmara University School of Medicine, Istanbul, Turkey.)
Contrast Enhancement
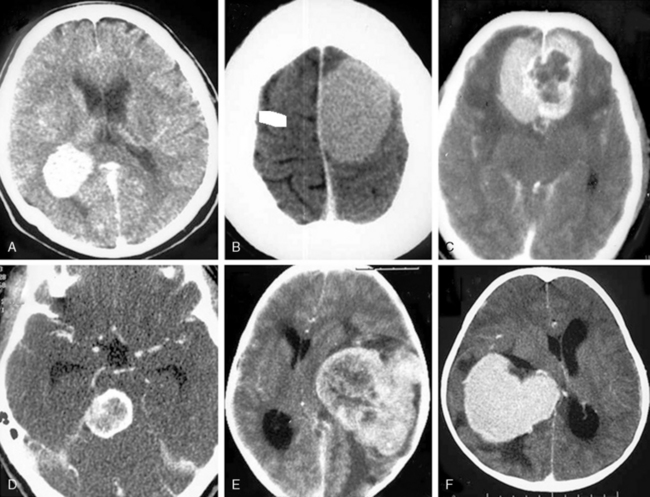
Meningiomas are extra-axial and very vascular tumors. On standard contrast-enhanced CT examinations, about 90% of meningiomas enhance strongly and homogeneously (Fig. 13-4A). Ten percent of meningiomas show mild contrast enhancement12 (Fig. 13-4B). Poor enhancement is commonly encountered in the microcystic histologic variant.15 Cystic meningiomas exhibit an enhancing cyst wall adjacent to the dura (Fig. 13-4C). Necrotic or totally calcified meningiomas enhance less strongly or the enhancement may only be detectable upon measurement of CT density units. Necrosis in meningiomas, which is more commonly seen in large or highly vascular meningiomas, may result in heterogeneous contrast enhancement (Fig. 13-4D, E). These large, hypervascular meningiomas are usually surrounded by capsule veins, which are seen as small, enhancing, round structures that can be traced into the tumor (Fig. 13-4F).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree