Chapter 17 Interferential current
INTRODUCTION
Interferential current (IFC) was developed in the 1950s and became increasingly popular in the UK during the 1970s (Ganne 1976). Although the actual definition of IFC has not been standardized in the literature, it can be described as the transcutaneous application of medium-frequency alternating currents, the amplitude of which is modulated at low frequency for therapeutic purposes. From this definition it can be seen that IFC is a form of transcutaneous electrical nerve stimulation (TENS).
IFC has been reported to reduce the skin resistance (and thus the discomfort) incurred by traditional low-frequency currents, while still producing low-frequency effects within the tissues (De Domenico & Strauss 1985, Low & Reed 2000). It has also been claimed to permit the treatment of deep tissues (De Domenico & Strauss 1985, Goats 1990, Hansjuergens 1986, Low & Reed 2000, Nikolova 1987, Willie 1969). Both of the above claims, which are unique to IFC, are largely unsubstantiated and have been challenged (Alon 1987, Palmer et al 1999a).
IFC has been reported to be available in between 77 and 98% of physiotherapy departments in Australia (Lindsay et al 1990, Robertson & Spurritt 1998), England (Pope et al 1995) and the Republic of Ireland (Cooney et al 2000). A strong relationship between the availability of IFC and its use has been established (Cooney et al 2000, Lindsay et al 1990, Robinson & Snyder-Mackler 1988), with 90% of physiotherapy clinicians with access to IFC reported to use it at least once per day (Lindsay et al 1990). Turner and Whitfield (1997) found that IFC was used by 43% of physiotherapists working in all clinical specialties in England. In terms of the conditions treated with IFC, 91% of respondents to one survey (Johnson & Tabasam 1998) used IFC to relieve pain. In a follow-up study (Tabasam & Johnson 2000), 26% of treatments were found to be for acute pain, 50% for chronic pain, and 16% for reduction of swelling. In another survey 88% of clinicians in the UK and Ireland reported using IFC to treat non-specific low back pain (Foster et al 1999). In addition to the treatment of pain IFC has also been used for other clinical conditions, including asthma (Emberson 1996), fractures (Fourie & Bowerbank 1997), incontinence (Laycock & Green 1988), psoriasis (Philipp et al 2000) and swelling (Christie & Willoughby 1990); and for accelerating tissue healing (Nikolova 1987), enhancing blood flow (Lamb & Mani 1994) and muscle strengthening (Bircan et al 2002).
These studies illustrate both a high rate of access to and use of IFC, at least in the UK, Ireland and Australia. It is interesting to note the prevalence of its usage for pain, indicating a perceived benefit to patients in terms of IFC-mediated effects on pain. Clinical trials of the effects of IFC on pain and other conditions, however, still remain scarce and largely inconclusive. Reports of the effectiveness of IFC have traditionally been scholarly statements in electrotherapy textbooks (De Domenico 1987, Kahn 1987, Nikolova 1987, Savage 1984) or largely descriptive journal articles (Belcher 1974, De Domenico 1982, Ganne 1976, Goats 1990, Willie 1969). The current emphasis on evidence-based practice, however, demands a more critical approach to clinical reasoning and treatment choices. This chapter will concentrate primarily on the literature related to IFC in the management of pain due to the prevalence of this use. Other claimed effects of IFC, such as on bone and tissue healing and muscle stimulation will be addressed in less detail.
PHYSICAL PRINCIPLES OF INTERFERENTIAL CURRENT
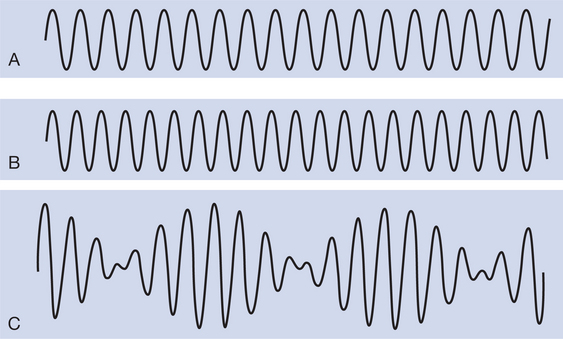
IFC describes a current applied at approximately 4000 Hz (usually referred to in physiotherapy as medium frequency) that rhythmically increases and decreases in amplitude at low frequency (adjustable between 0 and 200–250 Hz). IFC is produced by mixing two slightly out-of-phase medium-frequency currents, either by applying them so that they ‘interfere’ within the tissues or, alternatively, by mixing them within the stimulator prior to application (‘premodulated’ current). One current is normally of fixed frequency, for example at 4000 Hz, the other is adjustable, for example between 4000 and 4200 Hz. Theoretically, the two currents summate or cancel each other out in a predictable manner, producing the resultant amplitude modulated ‘interferential current’. The frequency of the resultant current will be equal to the mean of the two original currents and will vary in amplitude at a frequency equal to the difference between these two currents. This latter frequency is known as the ‘amplitude modulated frequency’ (AMF) or ‘beat frequency’. Figure 17.1 illustrates the production of IFC: two currents of 4000 and 4100 Hz are mixed, resulting in a medium-frequency current of 4050 Hz, which is amplitude modulated at a frequency of 100 Hz.
TREATMENT PARAMETERS AND METHODS OF APPLICATION
Amplitude modulated frequency
The AMF is traditionally considered to be the effective component of IFC, mimicking low-frequency currents and creating differential stimulation of nerve and tissue types (De Domenico 1982, Ganne 1976, Goats 1990, Low & Reed 2000, Nikolova 1987, Szehi & David 1980, Willie 1969). The theory of IFC is that the medium-frequency components simply act as ‘carrier’ currents, bringing the low-frequency AMF into the tissues (De Domenico 1982), where the body must be able to demodulate it.
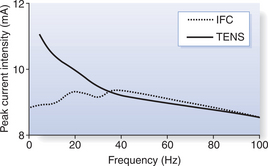
The mechanisms of this demodulation have not been established (Johnson 1999) and claims for the AMF to be the effective component of IFC have been challenged (Johnson 1999, Kinnunen & Alasaarela 2004, Martin 1996, Martin & Palmer 1995a, Palmer et al 1999a). It has been demonstrated that alteration of the AMF has little effect on the threshold activation of sensory, motor and pain responses (Kinnunen & Alasaarela 2004, Martin & Palmer 1995a, Palmer et al 1999a). IFC certainly did not follow the very clear frequency-dependent effects displayed by TENS, suggesting that the AMF does not, in fact, mimic low-frequency stimulation. In addition, the omission of an AMF (pure 4000 Hz current) displayed similar effects to when an AMF was used. It was concluded from this latter observation that the medium-frequency component of IFC, and not the AMF, was the dominant stimulating parameter (Palmer et al 1999a).
To illustrate this point, the mean sensory thresholds (the point at which the current was first reported as being perceived) from Palmer et al (1999a) are presented in Figure 17.2. These results can be explained by consideration of Table 17.1, which illustrates the effect of altering the AMF on the other components of IFC. This highlights that the resultant current frequency, and thus phase duration, change little. If the medium frequency is the main stimulating parameter then it is perhaps not surprising that the effect of the AMF might prove not to be as important as traditionally thought. However, it is obvious that the responses induced by IFC stimulation change with different AMF settings. Low AMFs, for example, elicit a ‘beating’ or ‘tapping’ sensation and muscle twitch responses, whereas higher AMFs elicit a ‘buzzing’ or ‘tingling’ sensation and a tetanic muscle contraction. This proposes some ability of the body to distinguish between high and low AMF settings. It has been found that participants experienced a 5 Hz AMF setting as being significantly more uncomfortable than either 50 or 100 Hz, with no significant difference between the level of discomfort at 50 and 100 Hz (Martin & Palmer 1996). In fact, at frequencies above approximately 40 Hz there seems to be little difference in the effects of IFC and TENS on somatosensory and motor thresholds (Palmer et al 1999a), with the comfort associated with muscle stimulation at 80 Hz also being demonstrated to be similar (Bircan et al 2002).
Table 17.1 Interferential current characteristics with a range of amplitude modulated frequencies (4000 Hz carrier frequency)
| Amplitude modulated frequency (AMF) | Resultant medium frequency (Hz) | Resultant medium frequency phase duration (μs) |
|---|---|---|
| 100 | 4050 | 123.5 |
| 40 | 4020 | 124.4 |
| 30 | 4015 | 124.5 |
| 20 | 4010 | 124.7 |
| 15 | 4007.5 | 124.8 |
| 10 | 4005 | 124.9 |
| 5 | 4002.5 | 124.9 |
| 0 | 4000 | 125 |
The AMF, at least in the lower frequency range (up to approximately 40 Hz) might therefore have a role in altering perceived comfort (Martin & Palmer 1996), but the main component of stimulation seems to be the medium frequency (Kinnunen & Alasaarela 2004, Martin & Palmer 1995a, Palmer et al 1999a). The AMF may be a synergistic partner, along with the medium frequency, but its role may be minor. As AMF selection has traditionally been a major component of clinical decision making with IFC, these observations have important significance.
AMF settings across a wide range between 1 and 130 Hz have been recommended in the literature for the treatment of pain, with little consensus. Clinically, however, the most popular (38% of replies) AMF used for pain relief was 130 Hz in the South West of Scotland (Scott & Purves 1991), although a ‘great range’ of AMF settings were used. In another survey of the AMFs used to treat a range of clinical conditions (Tabasam & Johnson 2000), it was reported that the mean was 85 Hz (range 1–150 Hz) when IFC was applied at a fixed frequency. In Northern Ireland, 70% of therapists used 80–120 Hz for the treatment of low back pain (Gracey et al 2001).
Frequency sweep
A frequency sweep is available on most IFC stimulators, where the AMF is altered over time. A sweep may be set between two prefixed AMFs, for example between 50 and 100 Hz. The pattern of change in frequency can also be adjusted on most machines. For example, it may be set to slowly increase and decrease over a period of 6 seconds (normally depicted by 6 6), or to give 1 second of stimulation at one frequency and then to automatically switch to the other frequency (1∫1). It has been reported that 96% of treatments by physiotherapists that employed a frequency sweep used a 6
6), or to give 1 second of stimulation at one frequency and then to automatically switch to the other frequency (1∫1). It has been reported that 96% of treatments by physiotherapists that employed a frequency sweep used a 6 6 pattern (Tabasam & Johnson 2000).
6 pattern (Tabasam & Johnson 2000).
A frequency sweep has been claimed to reduce adaptation (Low & Reed 2000, Nikolova 1987, Savage 1984), although evidence for the importance of this feature is, at best, only weak (Johnson 1999). One study, albeit small, demonstrated that the inclusion of a frequency sweep had no effect on the amount of adaptation experienced by participants (Martin & Palmer 1995b). This study requires replication, but empirical evidence for a frequency sweep reducing adaptation is certainly lacking.
A frequency sweep has also been claimed to allow stimulation of a greater range of excitable tissues (Low & Reed 2000, Savage 1984), thereby extending the scope of potential treatment effects. It has been found that cold pain threshold increased with a 6 6 sweep pattern compared to a 1∫1 pattern or sham stimulation (Johnson & Wilson 1997). Although the results of this study were not subjected to statistical analysis, it suggested a possible effect of frequency sweep. A later, larger study contradicted these results, however, finding no effect of frequency sweep (1∫1, 6∫6, 6^6, or burst) on cold-induced pain threshold, intensity and unpleasantness (Johnson & Tabasam 2003b).
6 sweep pattern compared to a 1∫1 pattern or sham stimulation (Johnson & Wilson 1997). Although the results of this study were not subjected to statistical analysis, it suggested a possible effect of frequency sweep. A later, larger study contradicted these results, however, finding no effect of frequency sweep (1∫1, 6∫6, 6^6, or burst) on cold-induced pain threshold, intensity and unpleasantness (Johnson & Tabasam 2003b).
FOUR-ELECTRODE AND TWO-ELECTRODE APPLICATION
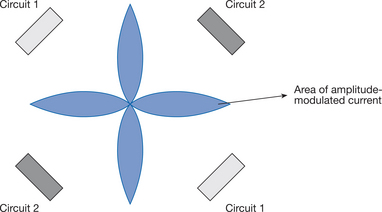
IFC may be produced either by applying the two medium-frequency currents via four electrodes so that they intersect in the tissues (sometimes called ‘quadripolar’ or ‘4 pole’ IFC), or alternatively by mixing the two currents in the stimulator prior to application via two electrodes (premodulated ‘bipolar’ or ‘2 pole’ method). It is claimed that a four-electrode application of IFC produces modulated current in a ‘clover-leaf’ pattern, as depicted in Figure 17.3, with the ‘leaves’ set up at right-angles to the two medium-frequency currents (Kahn 1987, Low & Reed 2000, Savage 1984).
It has been claimed that premodulated IFC displays a different distribution within tissues compared with four-electrode application (Hansjuergens 1986, Savage 1984). Whereas four-electrode IFC is claimed to be created deep within the tissues, premodulated IFC will be distributed similarly to conventional electrical stimulation (Savage 1984), with maximal current intensities underneath the electrodes, progressively decreasing with distance (Hansjuergens 1986). It has also been suggested that the wide dispersal of the area of interference with premodulated IFC might also reduce the effectiveness of treatment (Goats 1990). Such claims have been challenged.
Treffene (1983) found that there was a goodcorrelation between the expected and the actual pattern of IFC fields in a homogeneous water medium. The amplitude modulated current was set up not only in the central area between electrodes, however, but also underneath the electrodes. Lambert et al (1993), using computer models of a non-homogeneous medium and a human thigh, demonstrated that destructive interference (where the positive and negative charges of the two medium-frequency currents cancel each other out) was impossible with four-electrode IFC. Although some interference was evident, this varied at different positions in the thigh and there was no point at which there was zero resultant current. It was concluded that four-electrode applicationof IFC was fundamentally different from a two-electrode application, and that 100% modulation can only be obtained using two electrodes. Demmink (1995) directly measured the distribution of four-electrode IFC fields within pork tissue, discovering the current pattern and degree of modulation to be unreliable and haphazard. Additionally, the current did not follow a straight line between electrodes in each circuit. It must be concluded, therefore, that the pattern of IFC depicted in traditional textbooks is unrepresentative of that produced in biological tissue.
A haphazard distribution of modulated current, with modulation also occurring underneath the electrodes, seems to invalidate claims of the supremacy of four-electrode application. A premodulated application ensures that modulation is always 100% (Lambert et al 1993, Low & Reed 2000), although, as discussed, previously the AMF may not prove to be critical in any case. Ozcan et al (2004) found that four-electrode IFC was similar to two-electrode premodulated IFC in terms of the ratio between motor and sensory thresholds. Two-electrode IFC produced higher muscle torque values and less discomfort, however, suggesting that the two-electrode current was superior. A premodulated application has been found to be most commonly used by physiotherapists (79% of treatments) (Tabasam & Johnson 2000), although 80% of therapists have reported using four electrodes for the treatment of low back pain (Gracey et al 2001).
The use of two-electrode (premodulated) IFC is the only way to ensure 100% modulation and there is evidence that it may be more effective and comfortable in muscle stimulation. It also provides the easier alternative in a practical sense (De Domenico & Strauss 1985, Martin 1996). Evidence for differences in the effectiveness of two- and four-electrode IFC is embryonic, however. A four-electrode application can treat a larger area and, given that complete modulation may be relatively unimportant, this may present a valid treatment choice.
SUCTION AND PLATE ELECTRODES
IFC is often applied via electrodes that are held in place using an intermittent suction unit (with damp sponge inserts to ensure effective skin contact). Alternatively, flat carbon–rubber electrodes in damp sponge covers may be used. No literature has investigated the relative merits of either technique, although Tabasam and Johnson (2000) revealed that 90% of IFC treatments in their survey used carbon–rubber electrodes. Suction electrodes have been reported to have the advantage of allowing application to large flat areas or to patients who are relatively immobile (Savage 1984). The suction itself has also been claimed to stimulate cutaneous nerves and cause vasodilation (Low & Reed 2000). Such claims remain to be validated.
ELECTRODE PLACEMENT
For muscle stimulation, it has been recommended that a large electrode can be placed on the nerve supplying the muscle to be stimulated and another smaller electrode over the relevant motor point. Alternatively, two electrodes of equal size may be placed at the proximal and distal ends of the muscle (De Domenico & Strauss 1985). A process of trial and error may be used to find the placement that produces the strongest and most comfortable contraction. For pain relief, electrode placements may be used as for TENS (see Chapter 16). There is some preliminary evidence in people with low back pain (Hurley et al 2001) that application of IFC via electrodes placed over the spinal nerve root demonstrated better improvement in functional disability than when applied to the painful area (both were administered in combination with an evidence-based information booklet). There were no differences, however, between groups in terms of pain severity or overall health scores. For the treatment of low back pain, 80% of therapists have reported using electrode placements over the painful area and 50% over the spinal nerve root (Gracey et al 2001).
CURRENT INTENSITY
For pain relief, most authors advocate a current intensity that produces a ‘strong but comfort-able’ sensation (Goats 1990, Nikolova 1987, Savage 1984, Wadsworth & Chanmugam 1980). In an unpublished study, however, it was observed that the peak current intensity producing ‘strong but comfortable’ sensation in the forearm varied significantly between individuals and over time (Palmer 2002). Factors such as the area treated and the size and placement of electrodes will also determine the sensation induced by specific current intensities.
By definition, ‘strong but comfortable’ stimulation should be determined by participant report rather than peak current intensity settings. Intensity should be slowly increased until the patient signals that the required sensation has been reached. If used for muscle stimulation, the aim should be to use an intensity that produces a comfortable muscle contraction. Periodic adjustment of the intensity is recommended to compensate for any adaptation (Goats 1990, Robinson & Snyder-Mackler 1995, Savage 1984).
TREATMENT DURATION
Recommendations for treatment time have included 10–15 minutes with no longer than 20 minutes to one area (Savage 1984) or 10 minutes for most painful conditions (Wadsworth & Chanmugam 1980). Clinicians have reported actual treatment time to be between 11 and 15 minutes in the majority (61–72%) of cases (Tabasam & Johnson 2000, Gracey et al 2001, respectively). These treatment durations have no theoretical basis, however, and are more likely to be the result of practical constraints as opposed to scientific rationale (Johnson 1999). There is some evidence that IFC has short-lived effects, with raised experimentally induced cold pain thresholds returning to baseline levels within 10–20 minutes (Johnson & Tabasam 1999a, Johnson & Wilson 1997, Tabasam et al 1998). The applicability of such observations to the clinical situation continues to stimulate debate. However, if the reader accepts a fair degree of validity, then it may temper any expectancy of lasting pain relief following such short treatment sessions. This has yet to be specifically investigated clinically, however. The advent of small, portable IFC stimulators, as opposed to the traditional application via large, expensive and bulky stimulators in outpatient departments, is to be welcomed. These smaller stimulators will allow IFC to be used for longer periods, as recommended for TENS (McQuay et al 1997).
USE AS A TREATMENT ADJUNCT
It has been reported that IFC was used as an adjunct to other treatments in 73% of cases (Tabasam & Johnson 2000). Treatment combinations reported for low back pain have included exercise (90% of respondents), mobilization (80%) and manipulation (40%) (Gracey et al 2001). This suggests that IFC might not be seen as an effective treatment in its own right but rather as part of a comprehensive treatment programme. Turner and Whitfield (1997), in their multivariate analysis of techniques used by English physiotherapists, found that those who often used passive manipulation techniques were also high users of IFC and ultrasound. (Exercise therapy was commonly used by all therapists). Physiotherapy for painful conditions should incorporate a biopsychosocial approach (Martin & Palmer 2005) and, as such, it is important to elucidate the effects of IFC when combined with other treatments. Some of the investigations that have attempted to do so will be outlined later.
HAZARDS
A number of adverse effects have been reported with IFC treatment, including burns, increased pain, general malaise, nausea, vomiting, dizziness/faintness, migraine/headache and neurological effects (Kitchen 2000a, b, Partridge & Kitchen 1999). Very high current densities can produce a burn with any type of electrical current. This may occur where the effective size of the electrode is reduced by poor contact with the skin, and care must therefore be taken to ensure an even current density through equally damp electrode covers and good skin–electrode contact (CSP 1995). IFC should be incapable of producing chemical changes (and thus a chemical burn) as it is an evenly alternating current. Stimulation of the autonomic nervous system may account for some of the more general effects reported. At present, there are no adequate screening tools to identify patients who may experience undesirable reactions to IFC but close attention to equipment safety and servicing is of the utmost importance (CSP 1991). When applied to intact skin, decontamination of sponge electrode covers using hot water and detergent is recommended as an adequate infection control measure (Mercier & Haig 1993), although many centres use an antiseptic solution.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree