Fig. 1
Schematic diagram of the custom-designed restraint dish. Rose Bengal is intraperitoneally injected in zebrafish after anesthesia. The zebrafish is submerged in the Petri dish with MS-222 solution (75 mg/l) to maintain anesthesia. A rubber band (not shown here) is used to keep the zebrafish in upright position yet was loose enough to allow gill movement. Gentle perfusion of O2 is provided during the process to ensure adequate O2 supply
2,3,5-Triphenyltetrazolium Chloride (TTC) Staining and Quantification
The 2,3,5-triphenyltetrazolium chloride (TTC) staining method was adopted and modified from Preston et al. [9]. The brain slice was 1 mm thick of the optic lobe and was placed in TTC solution (2 % by weight) in the dark for 40 min at room temperature. After staining, the TTC solution was discarded and the slice was placed in a semi-micro cuvette with 400 μl DMSO/ethanol (1:1) solution in the dark for TTC extraction overnight. A spectrophotometer was used to measure TTC absorbance at 485 nm wavelength in each cuvette after slices were removed. Images of the brain slices were taken immediately after TTC staining and the volume of the brain slice was measured by software Motic plus 3.0.
Chemicals and Reagents
Ethyl 3-aminobenzoate methanesulfonate (MS-222), Rose Bengal, and 2,3,5-triphenyltetrazolium chloride (TTC) were purchased from Sigma Aldrich (St Louis, MO, USA). Activase®-tPA was purchased from Genentech (South San Francisco, CA, USA). All chemicals were dissolved in saline unless stated otherwise.
Statistical Analysis
Statistical analysis of percentage of recovery under different treatments was performed using Fisher’s exact test with R program. Statistical analysis of TTC measurements was performed using two-tailed Student’s t-test with Microsoft Excel. A value of p < 0.05 was considered significant.
Results
Zebrafish Exhibit Abnormal Behaviors after Photothrombotic Treatment
We placed the zebrafish in the Petri dish under anesthesia and placed the light probe on the skull in the region of optic tectum. The rest of the body was covered with aluminum foil. After 30 min of light exposure, the zebrafish was transferred to tank water to recover from anesthesia. Zebrafish regained normal swimming behavior after 15–30 min. We considered zebrafish managing normal and balanced swimming behavior as complete recovery from anesthesia. Zebrafish were kept in the dark. Only dim red light was used as necessary for observation, to prevent further excitation of Rose Bengal. We observed two types of abnormal swimming behavior by 24 h after photothrombotic treatment, rotating and circling (Fig. 2a, b). Rotating and circling were not observed in controls treated with Rose Bengal or light only. By 24 h after photothrombotic treatment, 96.8 % of zebrafish died or exhibited circling and rotating (Table 1). Our results indicate that photothrombosis produced delayed damage to zebrafish that was observed as behavioral change.
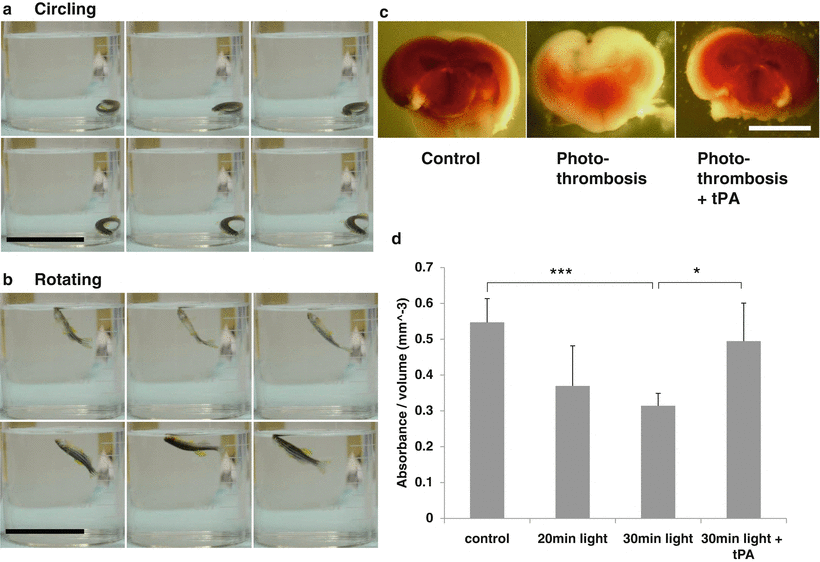

Fig. 2
Evaluation of brain damage after photo-thrombotic treatment. (a) Circling behavior after photothrombotic treatment. Scale bar: 5 cm. (b) Rotating behavior after photothrombotic treatment. Scale bar: 5 cm. Sequential images of zebrafish circling and rotating movements are acquired from 1 s of videos. (c) TTC staining of the zebrafish brain 24 h after photothrombotic treatment. Scale bar: 1 mm. (d) Spectrophotometric measurement shows that photothrombotic damage is light exposure dose-dependent, and treatment with tPA significantly improved TTC absorbance compared with the non-tPA treated group (* p < 0.05, *** p = 4.36587e−05, n = 7)
Table 1
Recovery rate between the tPA treated group and the non-tPA treated group after photothrombosis
Non-tPA | tPA | Fisher test | |
|---|---|---|---|
Total number | 31 | 20 | |
Number of death | 17 | 6 | 0.0673 |
Number of behavior change | 13 | 3 | 0.0642 |
Number of recovery | 1 | 11 | 3.359e−05 |
Photohrombotic Treatment Causes Quantitative Brain Damage
To confirm the brain damage, TTC staining was performed at 24 h after photothrombotic treatment. Zebrafish in the first control group were treated with Rose Bengal but not light exposed. The second control group was zebrafish receiving light exposure for 30 min, without Rose Bengal treatment. TTC staining and behavior observation indicated no brain damage from light exposure or Rose Bengal alone (data not shown). TTC staining of photothrombotic treated brain slices showed lack of staining in the area of light exposure, indicating ischemic cell death. Quantified measurement of TTC staining showed a significant reduction of brain cell viability with 20 min of light exposure, compared with the control group, and even greater reduction with 30 min of light exposure. Results also showed a strong correlation between abnormal swimming pattern and the extent of damage of the brain.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








