Fig. 1
Time course of plasma glyburide level following oral compared with intravenous bolus followed by 72 h infusion at 3 mg/day dose administration
Using an IV formulation of glyburide (RP-1127), a phase I trial in health human volunteers (ClinicalTrials.gov identifier: NCT01132703) evaluated the safety, tolerability, and pharmacokinetics of RP-1127. A total of 34 patients were tested at the following doses: 0.4, 3.0, 6.0, and 10.0 mg/day doses. Two patients (one each at 6 and 10 mg/day) discontinued the study because of persistent hypoglycemia. The incidence of blood glucose levels above 80 mg/dL was more frequent in the placebo group than in the 3 mg/day group, while there was no difference in the incidence of blood glucose levels below 70 mg/dL, implying a reduction in blood glucose at the 3 mg/day dose without hypoglycemia. There were no serious adverse events. Steady state plasma glyburide levels were determined to be 27.3 ng/mL. In preclinical experiments, a glyburide dosing regimen that was twice the effective dose average resulted in steady state plasma glyburide levels of 16 ng/mL. These observations suggest that the maximum tolerated dose of 3 mg/day results in human plasma levels that exceed the effective dose in rats.
The Glyburide Advantage in Malignant Edema and Stroke (GAMES) Pilot study [19, 20] (ClinicalTrials.gov identifier: NCT01268683) evaluated the safety and feasibility of administering a 3 mg dose of RP-1127 in patients with severe stroke at high risk for swelling. The primary objective of this prospective, open-label, phase IIa study was to assess the feasibility of enrolling, evaluating, and treating with RP-1127 (bolus followed by 72 h infusion at 3 mg/day dose) patients with severe ischemic stroke, whether or not they were treated with IV rtPA. Patients were enrolled at two centers, using the major inclusion criteria of clinical diagnosis of anterior circulation of ischemic stroke, baseline magnetic resonance imaging (MRI) diffusion weighted imaging (DWI) volume of 82–210 cm3, age 18–70 years of age, and start of drug infusion ≤10 h from symptom onset. The central inclusion criteria was a DWI lesion volume ≥82 cm3, chosen because of the high specificity with which it predicts malignant edema [20]. Patients with endovascular treatment for stroke, preexisting evidence of swelling, and recent use of sulfonylureas were excluded. In addition to the baseline MRI, patients underwent follow-up MRI at 72 h after drug infusion and clinical outcome assessment by modified Rankin Scale at 90 days. The mean age of enrolled patients was 51, the median baseline National Institutes of Health Stroke Scale (NIHSS) score was 18, and the mean baseline DWI lesion volume was 102 cm3. There were no serious adverse events related to the drug, including hypoglycemia. Figure 2 displays representative MRI brain images from a patient treated with RP-1127 (Panel a) and from an untreated historical patient (Panel b) at admission and 72–96 h. The edema and mass effect expected at 72 h are largely absent in the patient treated with RP-1127. Of the 10 patients, 1 patient had a neurological death, even after DC, and only 1 other patient required DC. Furthermore, 8/10 patients did not require any osmotherapy, intubation, or DC. In addition, there were no PH1/PH2 hemorrhages, despite 9/10 patients receiving IV rtPA. Neurological outcome at 3 months, overall mortality, need for DC, and hemorrhagic transformation were all improved compared with historical, matched patients [19–21]. GAMES-Pilot was a non-randomized, unblinded study with a small sample size; however, safety at the target dose of 3 mg/day and preliminary signals of efficacy provided the motivation for a randomized, double-blind phase II study.
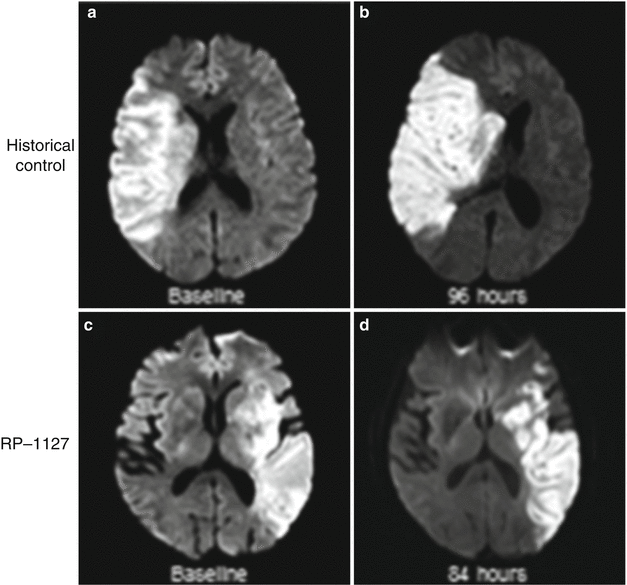

Fig. 2
Magnetic resonance diffusion weighted imaging at baseline (a, c) and at subacute follow up after stroke (b, d). The historical control (top panels) is from the EPITHET study. The bottom panels correspond to a GAMES-Pilot subject treated with RP-1127
Effect of RP-1127 on Intermediate Markers of Swelling
Previously, reliable, validated neuroimaging measures of brain swelling after stroke had not been established. Because the treatment effect of glyburide results, in part, from decreased edema formation, the application of a neuroimaging marker of edema is critical to assessing biological effect of the drug.
Our group initially investigated MRI-based ipsilateral hemisphere volumetric determination in stroke patients [22]. Serial MRI studies from stroke patients in the Echoplanar Imaging Thrombolytic Evaluation Trial (EPITHET) [23] with initial DWI lesion volume ≥82 cm3 were analyzed (baseline and day 3–5). The concordance correlation coefficient between readers was 0.90, and hemisphere volume correlated with early neurological deterioration (area under the curve 0.83; p = 0.04) Using this method, GAMES-Pilot subjects had an increase of 50 ± 33 cm3 compared with historical controls of 72 ± 27 cm3.
While serial change in hemisphere volume is a reasonable surrogate of swelling after ischemia, additional markers of cytotoxic and vasogenic injury can provide insight into the mechanism of action of RP-1127. MRI apparent diffusion coefficient (ADC) sequences vary depending on the restricted diffusion of water between the extracellular and intracellular compartments [24]. T2-based fluid attenuated inversion recovery sequences (FLAIR) may be sensitive to ischemic injury, which depends, in part, on the contribution of swelling from the vasculature [25]. To explore the possible effects of RP-1127 on these MRI signatures, serial MRI from GAMES-Pilot patients were compared with serial MRI from the control arm of the Normobaric Oxygen Therapy in Acute Ischemic Stroke Trial (NBO). Using a case-control design, ADC, FLAIR, and their respective signal intensity ratios (SIR) were obtained by normalizing the value within the stroke region of interest to the contralateral hemisphere.
The baseline characteristics for both groups were the same except more NBO subjects were male and a higher proportion of GAMES Pilot subjects underwent thrombolysis. There were no differences between ADC values or relative intensities in GAMES Pilot versus NBO subjects at baseline or day 1. In contrast, the FLAIR SIR in the NBO cohort was significantly higher as early as 24 h compared with GAMES Pilot subjects. The diminished increase in FLAIR SIR in RP-1127 treated GAMES Pilot subjects appeared to be a durable signal that lasted through the period of drug infusion, and there appeared to be a dose response relationship between the FLAIR ratio and RP-1127 level (p = 0.014). When patients were dichotomized at a plasma RP-1127 concentration that was associated with mild glucose lowering in the phase I study (25 ng/mL), the FLAIR SIR was lower (p < 0.01) in the group with high RP-1127 plasma levels (Fig. 3).
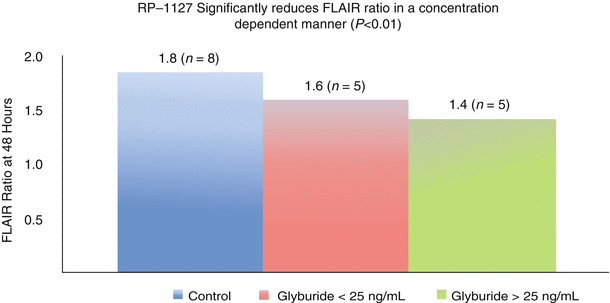

Fig. 3
Dose dependent relationship between RP-1127 and FLAIR Ratio
An analysis of FLAIR SIR in a cohort of acute stroke subjects suggested that FLAIR SIR was associated with symptomatic hemorrhagic transformation as well as matrix metalloproteinase-9 (MMP-9), an enzyme known to be associated with degradation of the blood-brain barrier, edema, and hemorrhagic transformation [26]. Increased levels of MMP-9 have also been associated with the administration of IV rtPA [27]. When MMP-9 levels were compared between RP-1127 treated patients and control large-stroke patients, glyburide treatment was associated with lower levels of MMP-9 [28]. There were no significant differences in the baseline characteristics, including exposure to rtPA.
These results have several important implications for RP-1127 translation. First, there are several candidate MRI signatures that may be modified by RP-1127 administration. In addition, plasma levels of MMP-9 may also decrease in association to RP-1127 exposure. Further, these changes may have a dose-response relationship. The potential biological response of these markers to RP-1127 is even more important considering their prior, established association with brain swelling and hemorrhagic transformation after ischemia, each of which RP-1127 proposes to reduce. The relationship between MRI signatures, MMP-9 levels, and RP-1127 will be further investigated in the ongoing GAMES-RP study; however, there is also an urgent need to further validate these relationships in independent populations of stroke patients, across a broad range of infarct volumes.
Phase II Randomized Trial
The robust preclinical and human data above directly led to the initiation of GAMES-RP (Clinicaltrials.gov identifier: NCT01794182), a randomized, multi-center, prospective, double-blind phase II trial in patients with a severe anterior circulation ischemic stroke who are likely to develop malignant edema. The study population consists of subjects with a clinical diagnosis of acute stroke with a baseline DWI lesion volume of 82–300 cm3, age 18–80 years, and time of symptom onset to start of drug infusion of ≤10 h. The primary objectives are to demonstrate the safety and efficacy of RP-1127 compared with placebo in patients with a severe anterior circulation ischemic stroke. The primary efficacy objective will be addressed by comparing the proportion of RP-1127 and placebo-treated patients with a day 90 modified Rankin Scale score ≤4 without decompressive craniectomy. The primary safety objective will be measured by comparing the frequency of adverse and severe adverse events in RP-1127 and placebo-treated patients. Subjects are randomized to either RP-1127 or placebo delivered as an IV bolus followed by an IV infusion for 72 h using the same dosing regimen as in GAMES-Pilot. In addition to a baseline MRI, subjects will undergo a 72–96 h follow-up MRI and serial clinical assessments through 1 year, aimed at assessing functional outcome, mood, and quality of life. The primary efficacy endpoint is at 90 days. A multidisciplinary team established clinical standardization guidelines, largely based on the American Heart Association guidelines for the management of patients with severe stroke [3, 29, 30]. This standardization of emergency and intensive care aims to make uniform practices surrounding sedation, use of osmotherapy and sodium management, fluid and insulin administration, and selection of patients for decompressive craniectomy. As of September 23, 2014, 52 subjects across 17 US hospitals have been enrolled, meeting the projected enrollment targets.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








