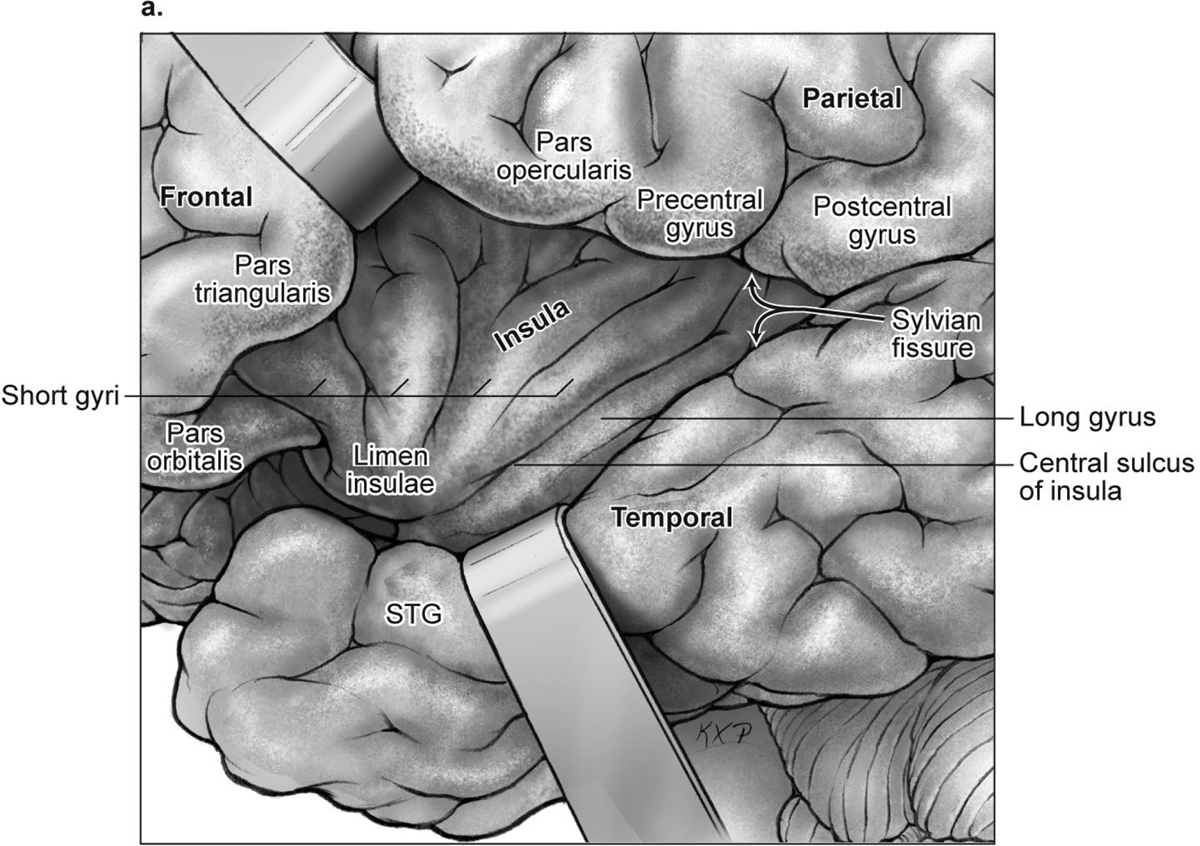
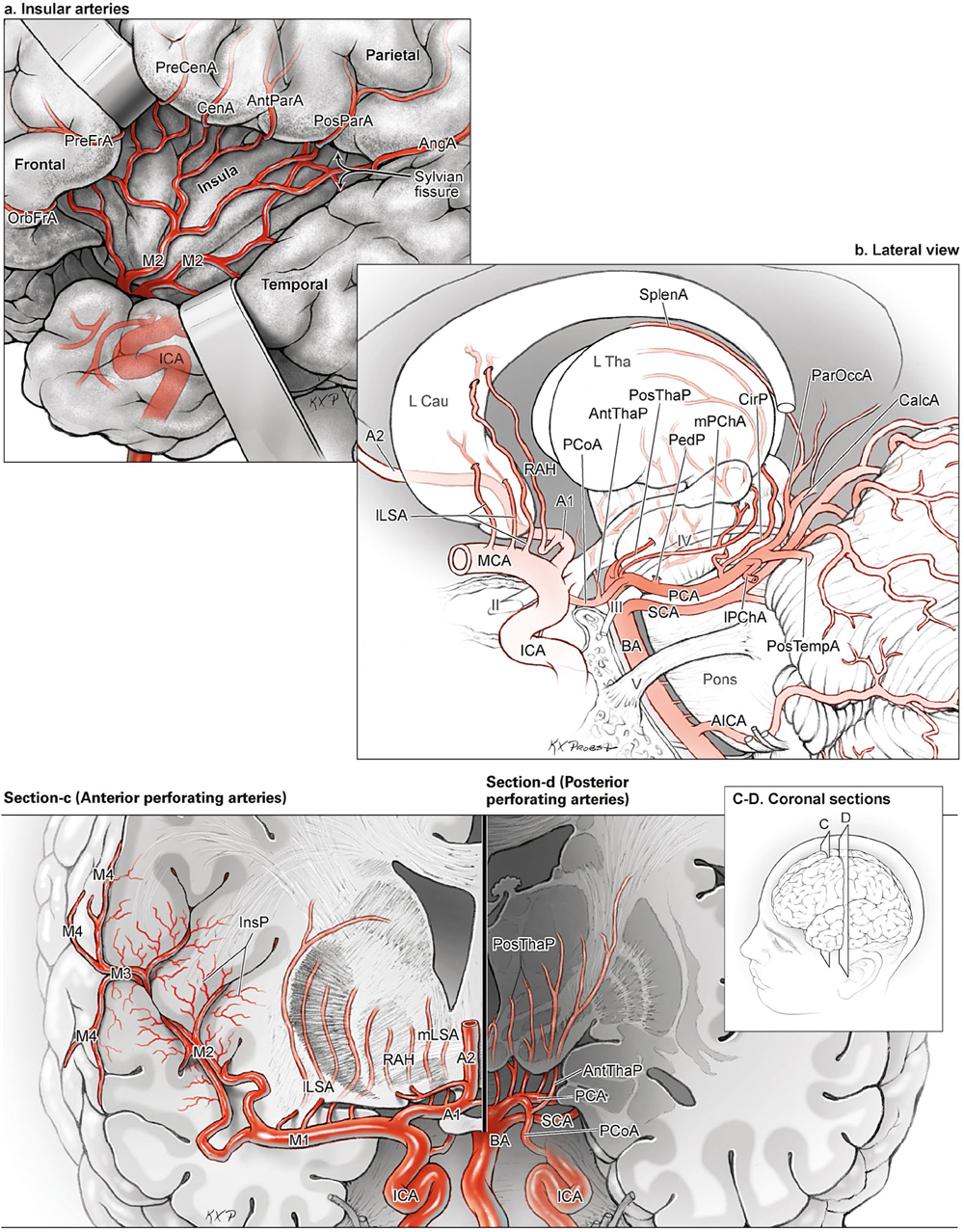
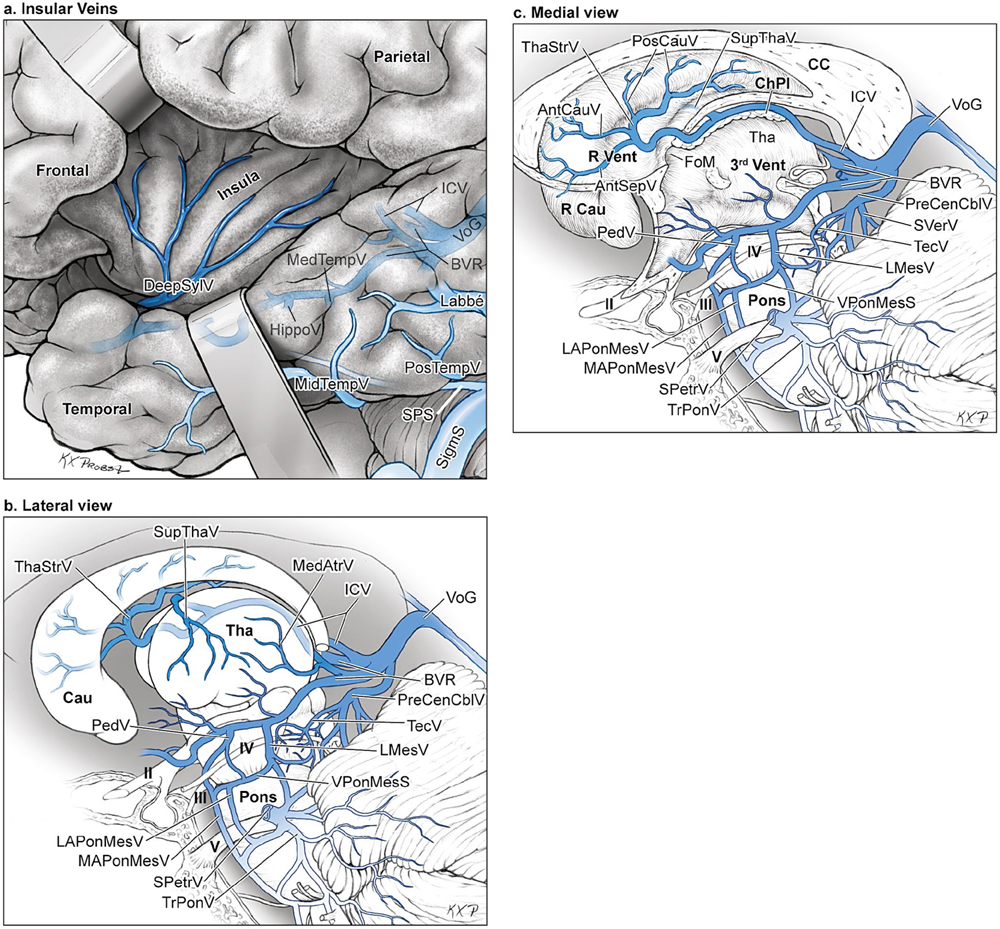
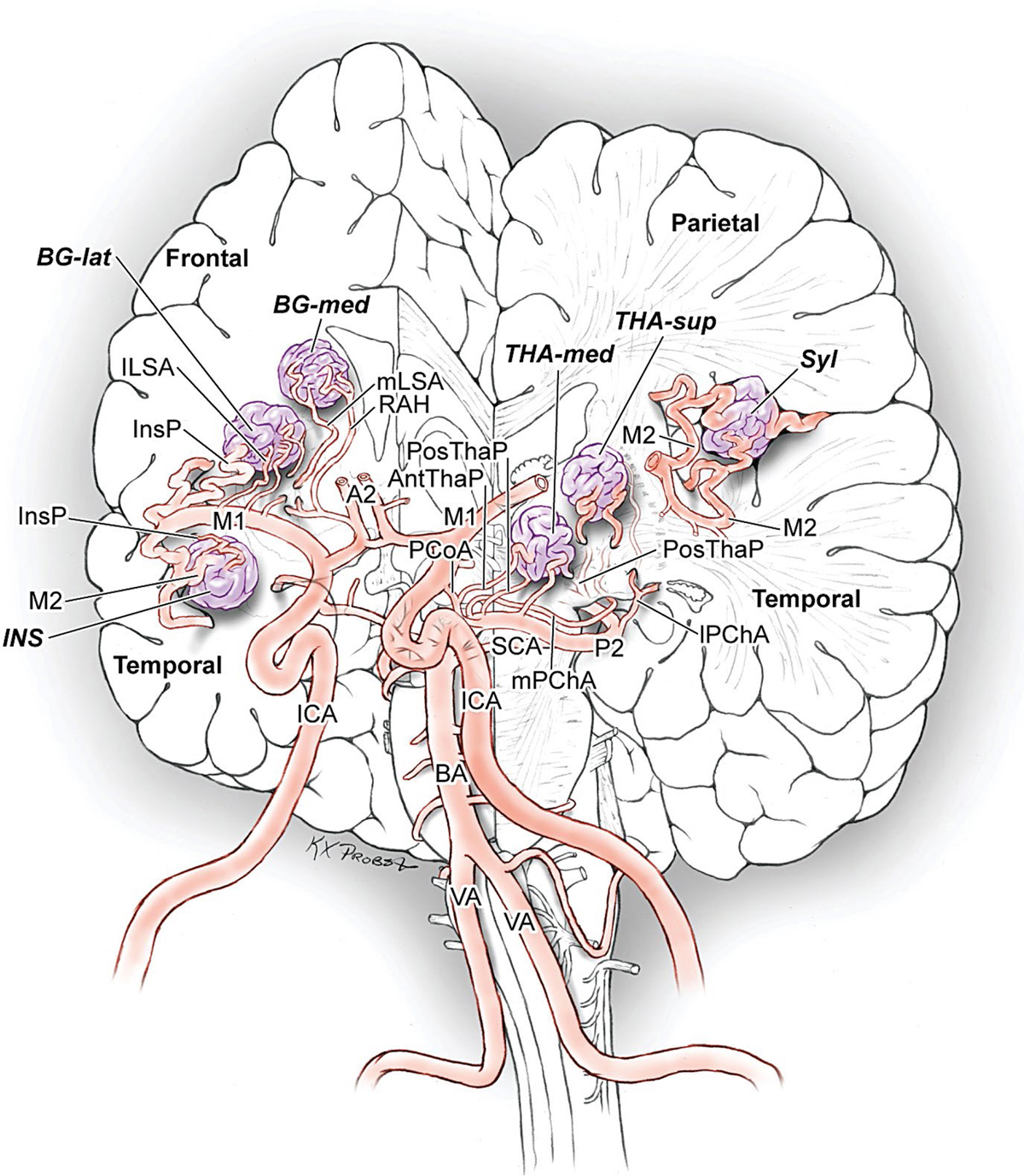
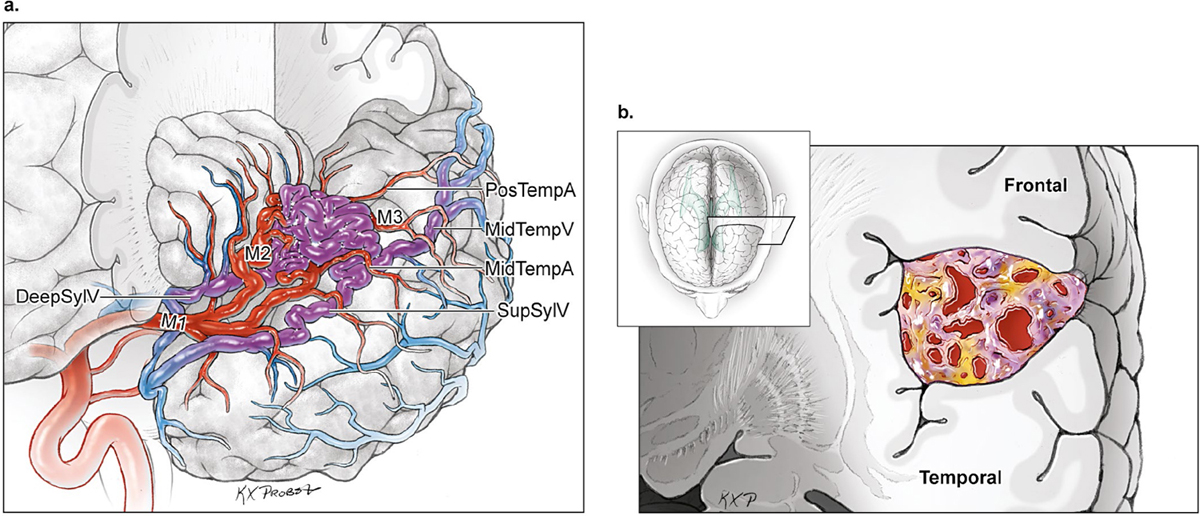
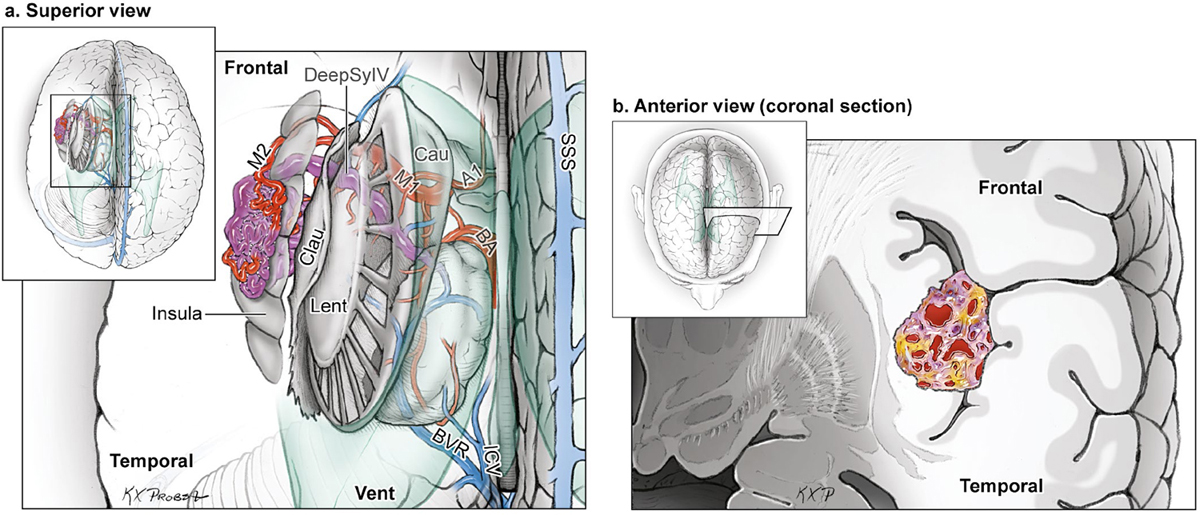
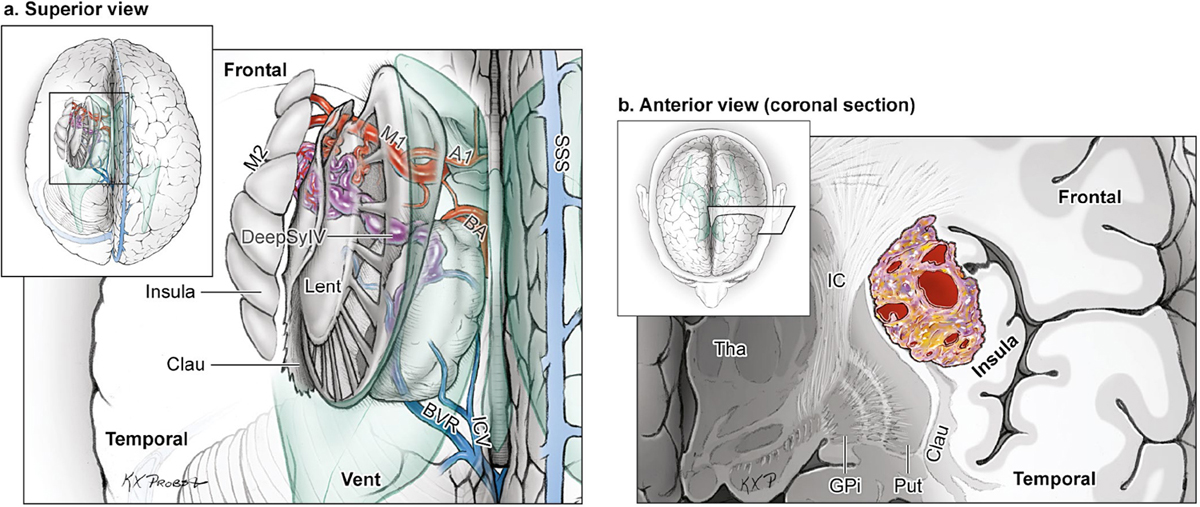
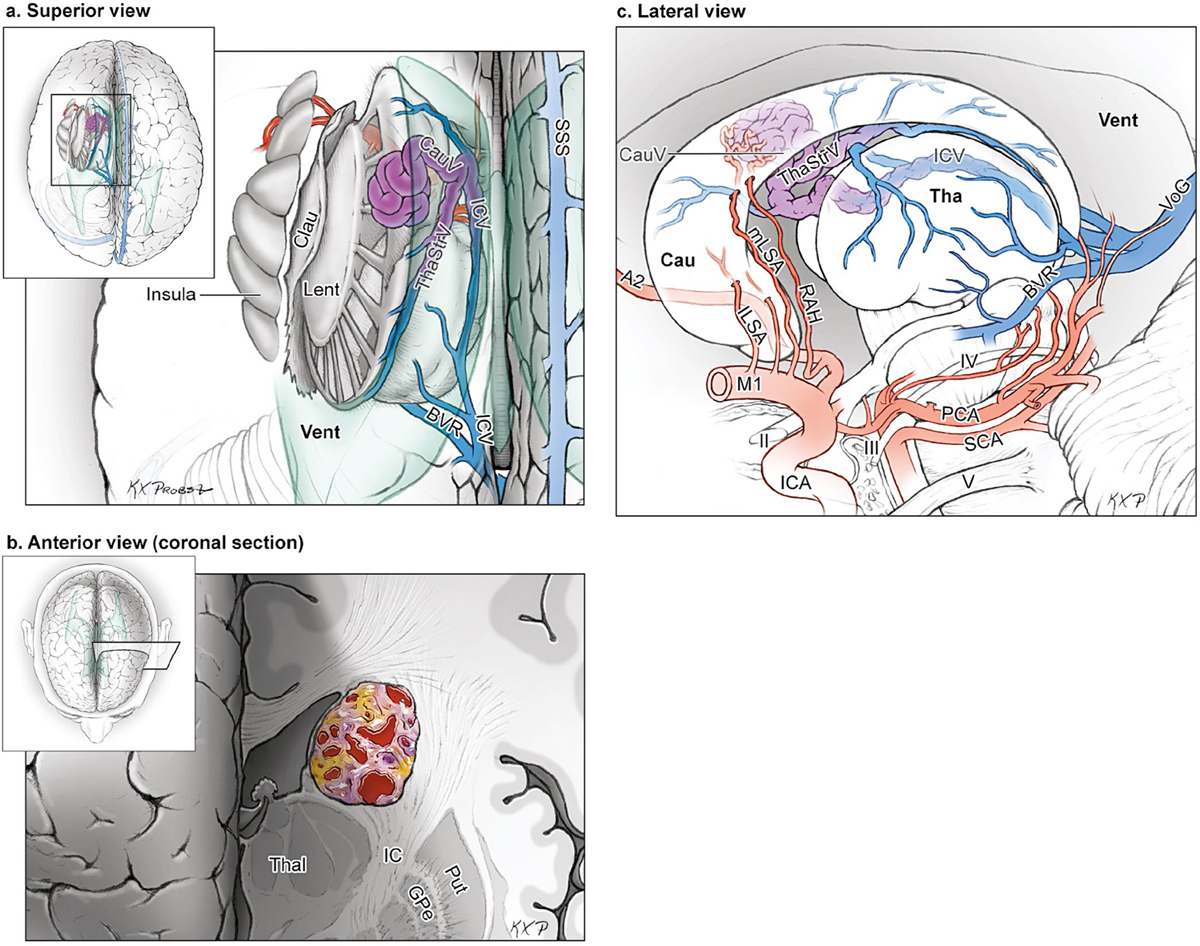
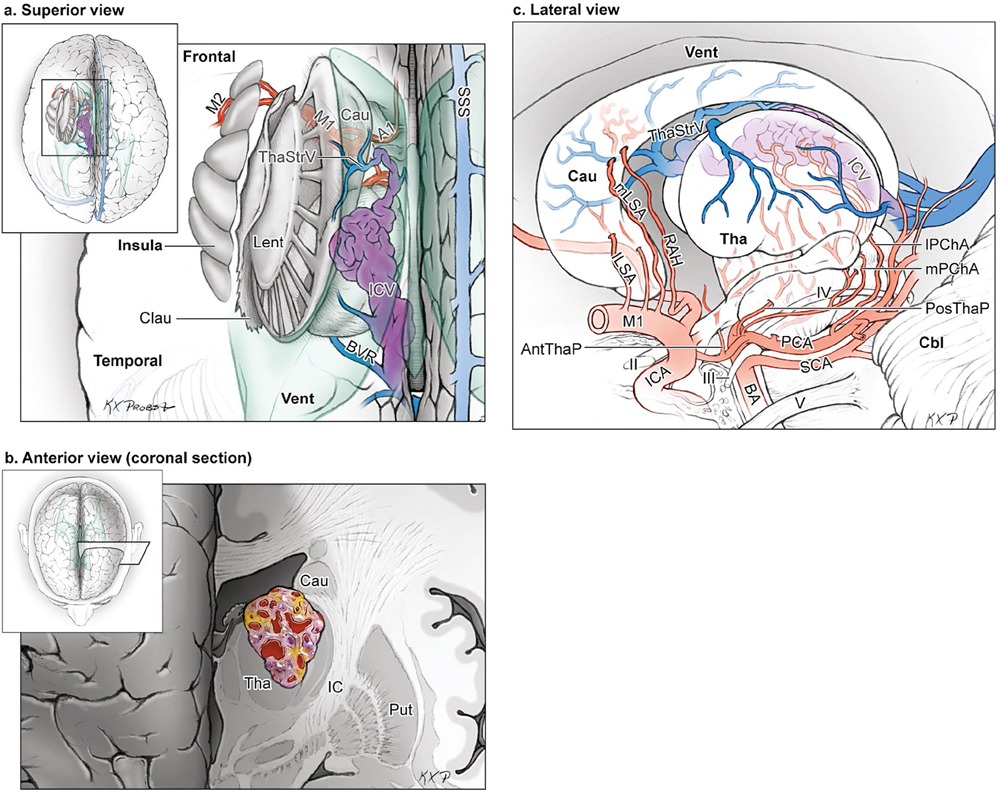
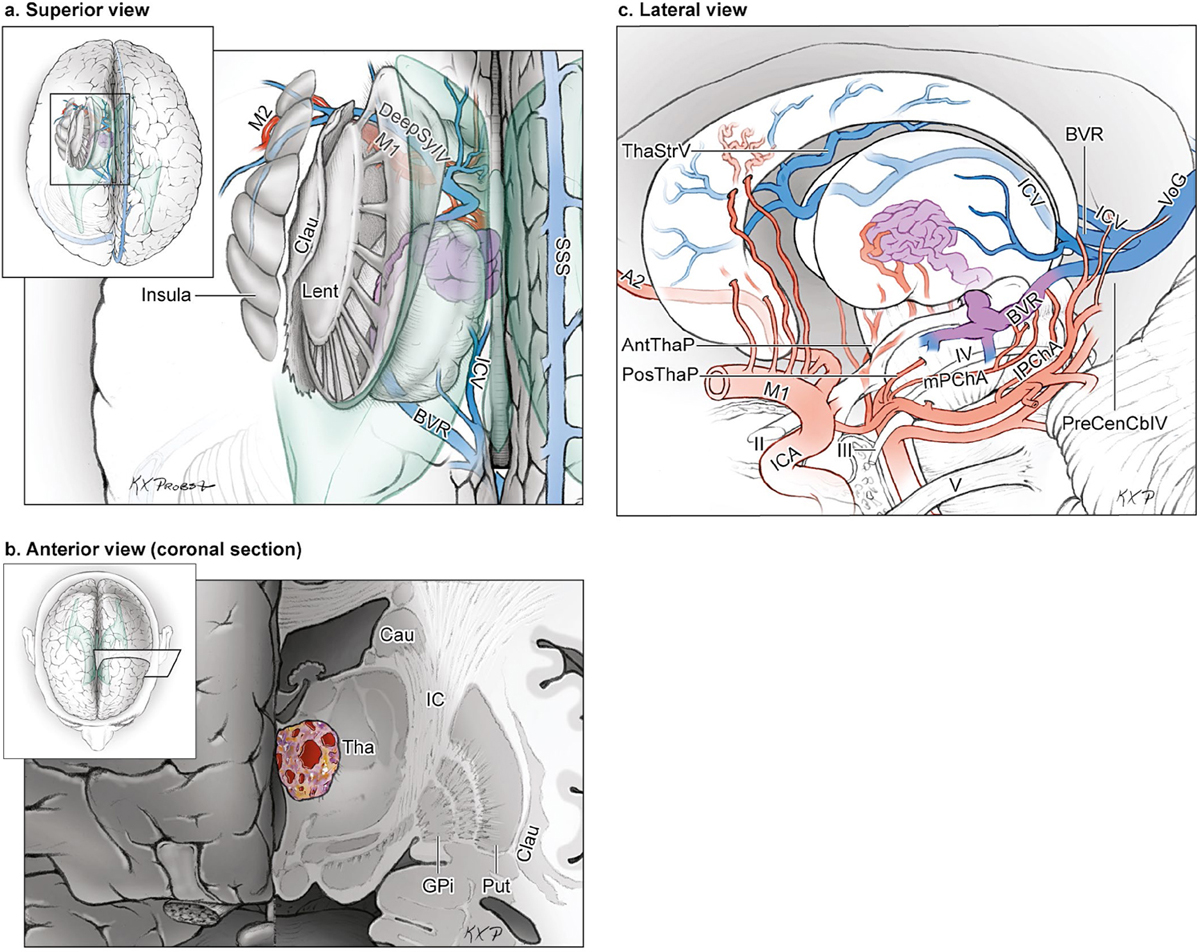
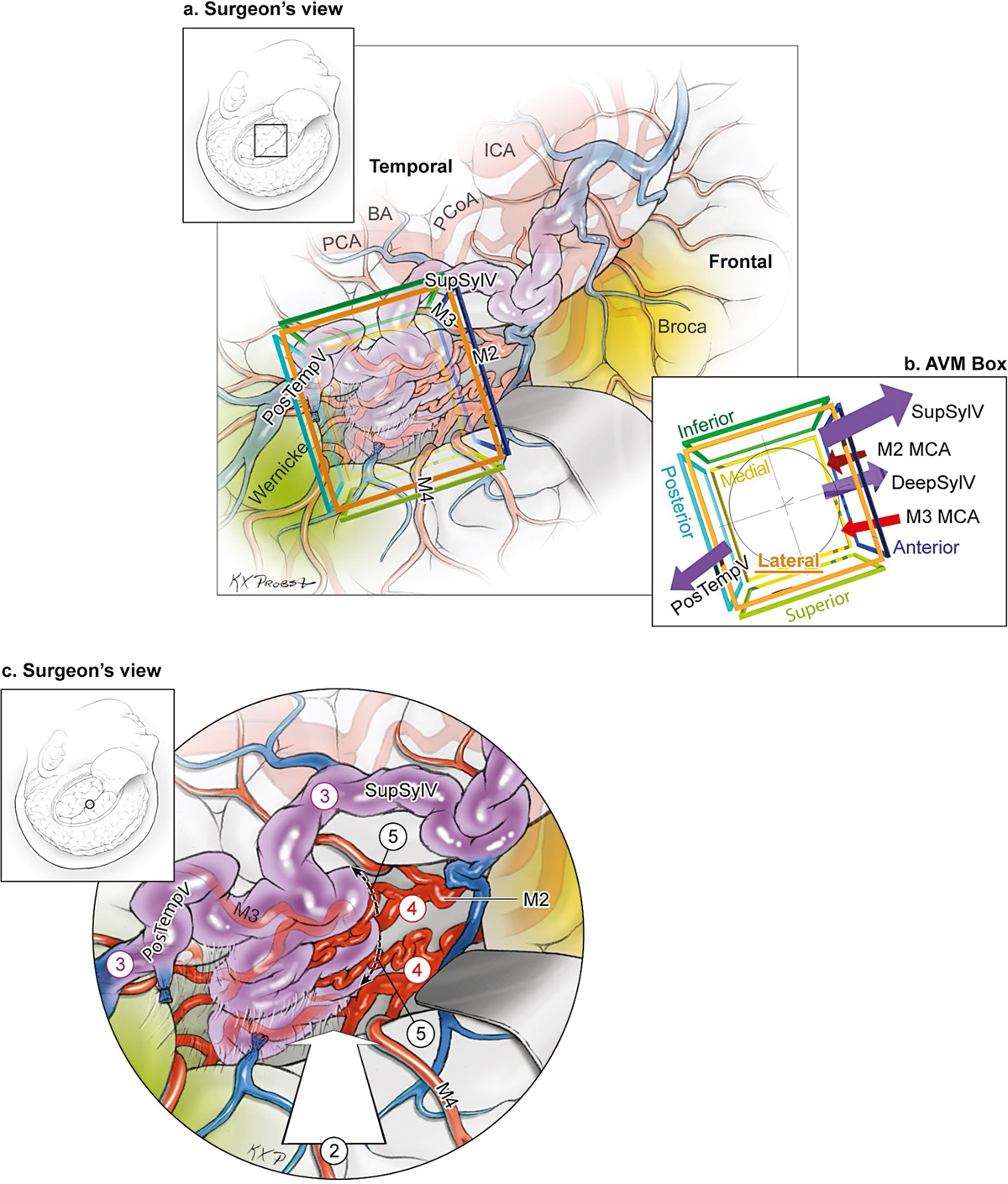
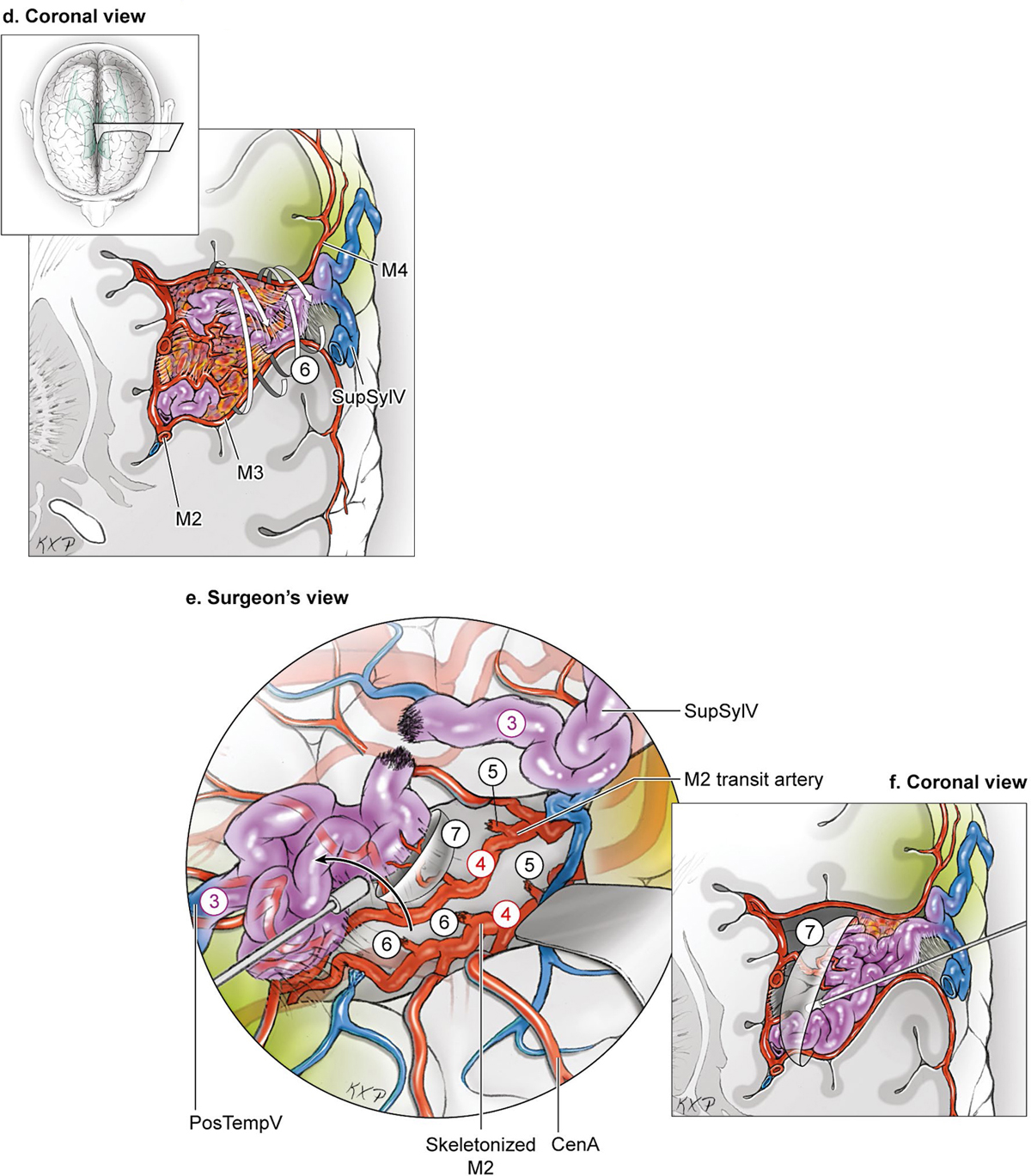
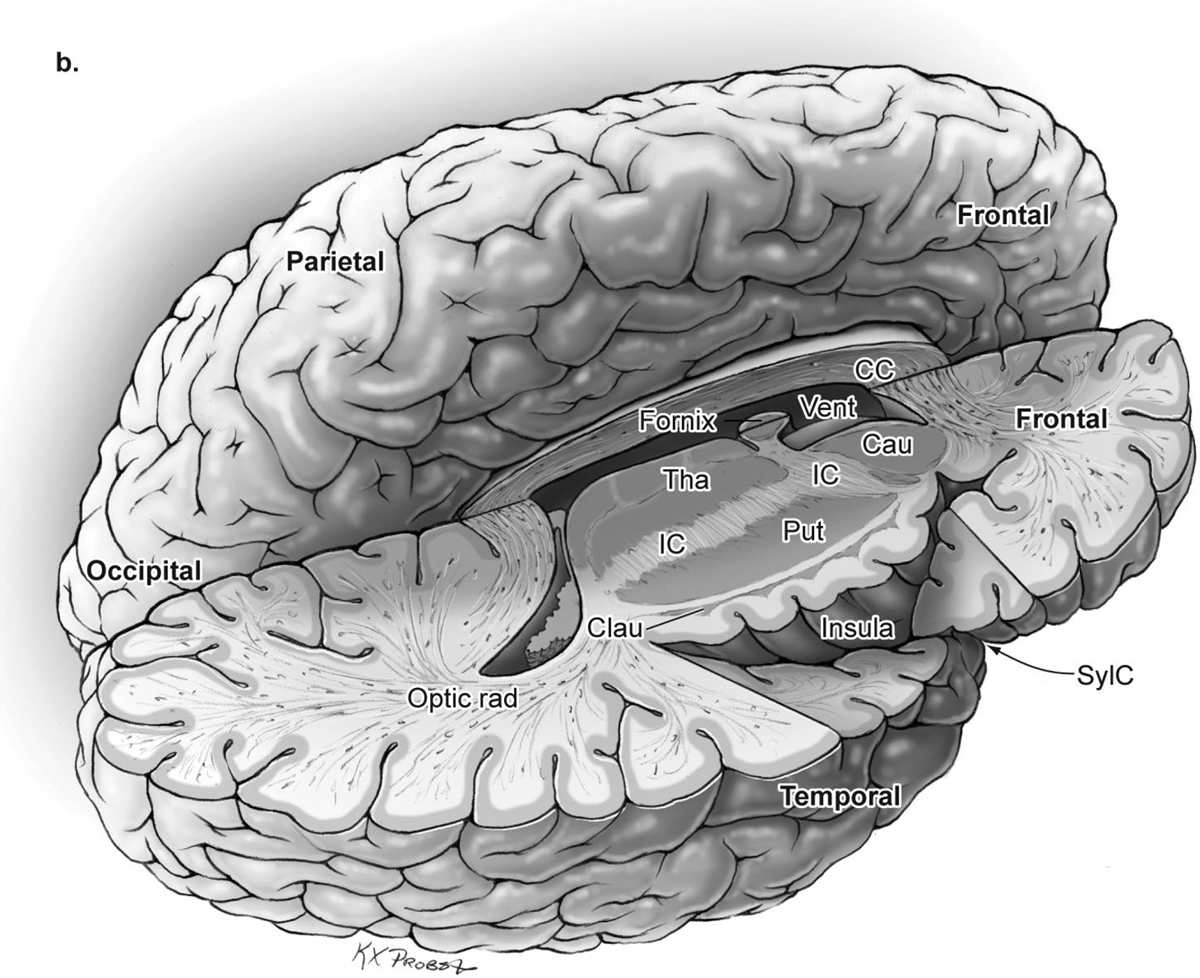
15 Deep Arteriovenous Malformations Deep arteriovenous malformations (AVMs) are situated in the insula and central core of the hemisphere. Although these areas lie beneath portions of frontal, temporal, and parieto-occipital lobes, they are discussed separately in this book from lobar AVMs because their depth and anatomy make them completely different surgically. Deep AVMs are discussed separately from ventricular AVMs because they are based in brain parenchyma rather than in ventricles. The insula is a triangular-shaped structure covering the lateral surface of the central core, with the triangle’s base under the frontal operculum and its apex directed anteriorly and inferiorly toward the limen insulae (Fig. 15.1a). The insula is divided into anterior and posterior parts by the central sulcus of the insula, which runs parallel and deep to the central sulcus of the convexity. The anterior insula is composed of three to five short gyri, and the posterior insula is composed of two long gyri. These gyri course radially from the limen insulae in a posterosuperior direction. The limen insulae, a slightly raised area overlying the uncinate fasciculus and the lateral border of the anterior perforated substance, marks the junction of the sphenoidal and insular compartments of the sylvian fissure, and the division between the M1 and M2 segments of the middle cerebral artery (MCA), where the artery turns upward. The insula is separated from the sylvian surfaces of the frontal, parietal, and temporal lobes by the limiting sulcus (circular sulcus). Fig. 15.1 (a) Microsurgical anatomy of the insula, as seen laterally through the left operculum. (b) Cross-sectional anatomy of the central core (horizontal section through the right hemisphere). The central core is situated between the insula and the midline, and is composed of the basal ganglia (putamen, globus pallidus, and caudate nucleus); thalamus; fornix; internal, external, and extreme capsules; and claustrum (Fig. 15.1b). The central core is attached to the rest of the hemisphere by an anterior and posterior isthmus. All information passing between the cortex and the brainstem and spinal cord is relayed in or carried by fibers passing through the central core. The central core and insula are supplied by perforating arteries: small end-arteries that terminate in critical deep structures (Fig. 15.2). Although enlarged cortical feeders to AVMs appear most threatening, they are superficial and often easy to identify, cauterize, and control. In contrast, small perforators that are often overlooked angiographically are deep and difficult to cauterize; they traverse vital white matter tracts, persistently supply the nidus long after the cortical feeders are interrupted, and are accessible only after arriving at the last margin of the AVM. Therefore, they warrant special respect. The lateral lenticulostriate arteries (lLSA) are the MCA’s perforators. On average, 10 perforators originate from the M1 segment along its superior-posterior surface proximal to its bifurcation, although an early bifurcation results in their postbifurcation origin. The lLSA penetrates the brain at the anterior perforated substance, passes through the putamen, and supplies the upper part of the internal capsule, globus pallidus, and caudate nucleus (head and body). The medial lenticulostriate arteries (mLSA) are the anterior cerebral artery’s (ACA) perforators. On average, eight perforators arise from the A1 segment, concentrated more proximally (laterally) than distally (medially). The mLSA enters the anterior perforated substance to supply the suprachiasmatic part of the hypothalamus and third ventricle, as well as the dorsal surfaces of the optic chiasm, nerve, and tract. The recurrent artery of Heubner (RAH) is the most medial mLSA, originating from the A1-A2 ACA junction directly lateral to the anterior communicating artery (ACoA) and slightly on the A2 side. It doubles back on itself, paralleling the A1 segment and coursing above the internal carotid artery (ICA) terminus and M1 MCA to the proximal sylvian fissure. It enters the anterior perforated substance with other lLSAs to supply the head of caudate nucleus and adjacent internal capsule, and may also supply the anterior putamen and globus pallidus. Fig. 15.2 (opposite) (a) Insular arteries from MCA trunks are unnamed stem arteries (M2 segments) that acquire names when they reach the cortical surface (M4 segment). (b) The central core is supplied by perforating arteries, including lenticulostriate arteries (lLSA, mLSA, and RAH), insular perforators (InsP), thalamoperforators (AntThaP and PosThaP), thalamogeniculate arteries (ThGenP), peduncular perforators (PedP), and circumflex perforators (CirP) (lateral view). (c) Perforating arteries from the anterior circulation and (d) the posterior circulation (coronal cross-sectional views through the basal ganglia and thalamus, respectively). Anterior thalamoperforators (AntThaPs) originate from the posterior communicating artery (PCoA) along its superior and lateral surfaces. There are, on average, eight anterior thalamoperforators, the largest of which is the premammillary artery, which penetrate the third ventricle in front of or beside the mammillary bodies. AntThaP supplies the posterior hypothalamus, ventral thalamus, optic tract (anterior third), posterior limb of internal capsule, posterior perforated substance, and subthalamic nucleus. Posterior thalamoperforators (PosThaPs) originate from the P1 segment of the posterior cerebral artery (PCA), typically from its middle third along the superior and posterior surfaces. The number of thalamoperforators varies from one (artery of Percheron) to 12, with an average of four. PosThaP ascends to the posterior perforated substance and the upper part of the interpeduncular fossa, and supplies the thalamus, posterior hypothalamus, subthalamus, and the medial part of midbrain. Peduncular perforators (PedPs) arise from the P2 segment and pass directly into the cerebral peduncle, supplying corticospinal and corticobulbar tracts, substantia nigra, red nucleus, and tegmentum. Circumflex perforators (CirPs) encircle the brainstem variable distances (short and long) before penetrating the brain. They arise from the P1 and P2 PCA segments and course around the midbrain medial to the parent PCA. The short CirP penetrates at or before the geniculate bodies, and the long CirP travels back to the quadrigeminal cistern to supply the collicular plate, superior colliculi primarily. The anterior peduncle is typically supplied by the PedP whereas the lateral peduncle is supplied by the CirP. Thalamogeniculate arteries (ThGenPs) originate from the P2 PCA beneath the lateral thalamus and ascend to the geniculate bodies in the roof of the ambient cistern (AmbC). On average, two or three ThGenPs arise at the P2A-P2P junction to supply the posterior half of the lateral thalamus, posterior limb of internal capsule, and optic tract. The deep venous system collects from veins in the basal cisterns and ventricles, which were discussed in Chapter 14. Important veins include the lateral ones draining the ventricular surface of the central core (Fig. 15.3): the anterior and posterior caudate vein (CauV) draining the caudate nucleus and terminating in ThaStrV; ThaStrV coursing in the striothalamic sulcus between the caudate nucleus and thalamus; the internal cerebral vein (ICV), a paired vein running in the velum interpositum from the foramen of Monro (FoM) to the vein of Galen (VoG); the VoG, which collects from the two ICVs, two basal veins of Rosenthal (BVRs), and the precentral cerebellar vein; and lateral atrial vein (AtrV), which drains the lateral wall of the atrium, posterior thalamus, and caudate body, and then passes through the choroidal fissure to reach the BVR in the ambient or quadrigeminal cisterns. The insula is drained by insular veins, which collect in DeepSylV and the BVR. Fig. 15.3 (a) Insular veins collect in the deep sylvian vein and BVR (lateral view through operculum). (b) Veins draining the left central core (lateral view). (c) Veins draining the right central core and right ventricular system (medial view with left central core removed). Deep AVM subtypes include the pure sylvian, insular, basal ganglial, and thalamic AVMs (Fig. 15.4). The pure sylvian AVM, as introduced by Sugita in his classification of AVMs in the sylvian fissure, is located in the fissure without a parenchymal base on the frontal, temporal, or insular cortex (Fig. 15.5). Unlike most other AVMs that are based in brain tissue, pure sylvian AVMs are entirely in the subarachnoid space. The AVM splays the fissure, displacing the superior temporal gyrus down on the temporal side and the precentral gyrus, pars opercularis, and pars triangularis up on the frontal side. There are few other such AVMs whose parenchymal planes are replaced by arachnoidal planes: VoG AVMs, pial brainstem AVMs, and some ventricular AVMs. Pure sylvian AVMs are particularly clean because the dissection is subarachnoid without pial or parenchymal transgression. Furthermore, the location is superficial with excellent exposure. Pure sylvian AVMs are supplied by MCA stem arteries arising from both superior and inferior trunks along their M2 and M3 segments. The nidus resides laterally in the operculum, which situates feeding MCA branches deep to the nidus. Feeders are typically large terminal arteries with high-flow intranidal shunts, which frequently enlarge draining veins into varices. SupSylV and DeepSylV provide drainage, with superficial veins more dominant than deep veins. The sylvian AVMs that do not respect the opercular surfaces of the frontal lobe, temporal lobe, or insula are categorized as sylvian frontal, sylvian temporal, or insular AVMs, respectively. Pure sylvian AVMs were the least common of the deep AVMs (14%), which attests more to their rarity than their operability. Fig. 15.4 Overview of deep AVM subtypes, which include pure sylvian (SYL), insular (INS), basal ganglial [lateral (BG-lat), and medial (BG-med)], and thalamic [superior (THA-sup) and medial (THA-med)], as seen in an anterior oblique, coronal cross-sectional view. Fig. 15.5 The pure sylvian AVM subtype, viewed anteriorly (a) with the frontal and parietal lobes removed partially, and (b) in coronal cross section. This AVM is located in the subarachnoid spaces of the sylvian cistern without a parenchymal base in the frontal, temporal, or insular cortex. It is supplied by MCA stem arteries (M2 and M3 segments) and drained by sylvian veins (SupSylV and DeepSylV). Insular AVMs sit in the limen insulae, short gyri, and long gyri on the insular surface, but lateral to the basal ganglial structures and respectful of the claustrum (Fig. 15.6). Insular AVMs were included in Sugita’s classification of sylvian fissure AVMs and described as deep sylvian AVMs. Insular AVMs were the most common of the deep AVM in my practice, accounting for nearly half (45%) of those treated surgically. The M2 segments supply insular AVMs and course above or lateral to the nidus, requiring the resection to proceed between these arteries. Feeders are large stem arteries and the InsP. Sylvian veins drain these AVMs, with DeepSylV more dominant than SupSylV. Although the role of the insular cortex in speech processing and in conduction between Wernicke’s and Broca’s areas is unclear, it is considered non-eloquent. However, insular AVM exposure in the dominant hemisphere requires dissection adjacent and deep to speech areas in both the frontal and temporal lobes. Fig. 15.6 The insular AVM subtype: (a) superior and (b) coronal cross-sectional views. This AVM is located in insular cortex, supplied by MCA stem arteries (M2 segment) and insular perforators (InsP), and drained by DeepSylV. The basal ganglia AVM is deep to the insular cortex in the putamen (Fig. 15.7), globus pallidus, anterior limb of the internal capsule, or caudate nucleus (Fig. 15.8). It sits lateral to the posterior limb of the internal capsule, whereas the thalamic AVM sits medial to the posterior limb. Basal ganglial AVMs have no cortical representation whatsoever and can be reached only laterally through a nonanatomic, transcortical route created either surgically or by hemorrhage. Consequently, basal ganglial AVMs located laterally in the putamen are the most favorable for resection (lateral variant). Some located in the caudate head can be reached medially through a transependymal route through the lateral ventricle (medial variant). The AVMs in other basal ganglial locations are less favorable for surgery. Lateral basal ganglial AVMs are deeper than insular AVMs and are fed by the InsP and lLSA rather than MCA stem arteries. Medial basal ganglial AVMs receive inputs from the mLSA and RAH. Drainage collects in the deep system via DeepSlyV (lateral variant) or CauV and ThaStrV (medial variant). Basal ganglial circuits are eloquent, and proximity to the internal capsule makes these AVMs dangerous. Fig. 15.7 The basal ganglial AVM subtype in the putamen: (a) superior and (b) coronal cross-sectional views. This lateral variant is located beneath the insular cortex in the putamen or globus pallidus, supplied by lLSA and InsP, and drained by DeepSylV. Fig. 15.8 The basal ganglial AVM subtype in the caudate nucleus: (a) superior, (b) anterior (coronal cross section), and (c) lateral views. This medial variant is located beneath the lateral wall of the lateral ventricle in the caudate nucleus, supplied by mLSA and RAH, and drained by CauV. Together with the hypothalamus and brainstem, the thalamus is one of the inviolable brain structures that inspires caution. Compelling indications are needed to operate on a thalamic AVM: hemorrhage, significant preexisting deficits, failure of stereotactic radiosurgery, and young age. Thalamus does not tolerate much transgression, and operable AVMs must be small and superficially located on surgically accessible surfaces. Two such surfaces are (1) the superior thalamic surface that forms the floor of the ventricular body, behind the FoM, between the body of the fornix and the body of the caudate nucleus, and deep to the choroid plexus (superior variant, Fig. 15.9); and (2) the medial thalamic surface that forms the lateral wall of the third ventricle deep to the FoM (medial variant, Fig. 15.10). These surfaces are accessible through transcallosal and transcallosal-transchoroidal fissure approaches, respectively. Importantly, AVMs in these entry zones avoid the medial knee of internal capsule. Thalamic AVMs are supplied from below by AntThaP (from PCoA) and PosThaP (from P1 PCA), and from above by lPChA and mPChA. Thalamic AVMs on the superior wall drain ventricularly to the ICV, whereas medial wall drain cisternally to the BVR. Fig. 15.9 The superior thalamic AVM subtype, viewed (a) superiorly, (b) anteriorly (coronal cross section), and (c) laterally. This superior variant is located on the superior thalamic surface, supplied by PosThaP and the posterior choroidal arteries (mPChA and lPChA), and drained by the ICV. Fig. 15.10 The medial thalamic AVM subtype, viewed (a) superiorly, (b) anteriorly (coronal cross section), and (c) laterally. This medial variant is located on the medial thalamic surface, supplied by thalamoperforators (AntThaP and PosThaP), and drained by the BVR. Pure sylvian AVMs are exposed with a pterional craniotomy and transsylvian approach (Fig. 15.11). The patient is positioned supine with the head turned 20 to 30 degrees away from the AVM and extended 20 degrees, as for an MCA aneurysm, allowing the frontal and temporal lobes to fall naturally to either side of the fissure like pages in a book resting on its binding. The scalp incision and craniotomy extend around the posterior AVM margin, which might require a hairline incision and standard pterional craniotomy for anterior AVMs and a question mark incision and additional parietal/posterior temporal expansion for posterior AVMs (step 1). Pure sylvian AVMs are exposed and circumdissected in the subarachnoid phase of dissection without pial, parenchymal, or ependymal dissection. Sylvian fissure arachnoid is incised below the pars triangularis where the distal fissure is widest, SupSylV is mobilized to the temporal side of the fissure, and the fissure is split from distal to proximal (step 2). Drainage through an enlarged or variceal SupSylV remains centered over the nidus throughout the dissection (step 3). Supply originates from the insular M2 and opercular M3 segments of MCA stem arteries and from both superior and inferior trunks (step 4). These feeding arteries are situated underneath the nidus on both the frontal and temporal sides, forming an anteromedial front (step 5). Sylvian arteries are a mixture of terminal feeding arteries, transit arteries, and bystander arteries. High-flow terminal feeders are identified in the proximal fissure on the inferomedial AVM margin as large pipes that frequently require occlusion with aneurysm clips rather than cautery. Transit arteries require skeletonization in a distal-to-proximal direction to interrupt their smaller nidal inputs and also preserve cortical arteries that continue en passage. Uninvolved bystander arteries are separated from the nidus and mobilized temporally or frontally. Meticulous arachnoidal dissection in the sylvian cistern maintains pial integrity and no parenchymal transgression is needed for circumdissection (step 6). Deep dissection exposes the full course of the M2 and M3 stem arteries, DeepSylV drainage, and insula cortex (step 7). Pure sylvian AVMs are extraparenchymal and therefore non-eloquent, but are adjacent to speech areas in the dominant frontal and temporal lobes (Figs. 15.12 and 15.13). Fig. 15.11 Resection strategy for pure sylvian AVMs. (a) Step 1, exposing the AVM with a pterional craniotomy (surgeon’s view). (b) AVM box showing arterial inputs anteriorly and medially, and eloquence anteriorly (Broca’s area) and posteriorly (Wernicke’s area). (c) Step 2, splitting the sylvian fissure; step 3, mobilizing superficial draining veins temporally; step 4, following M2 stem arteries to the AVM; and step 5, dividing the anteromedial front. (d) Step 6, circumdissecting within the subarachnoid spaces of the sylvian fissure (coronal cross-sectional view). (e) Step 7, dividing terminal M2/M3 feeding arteries, skeletonizing stem arteries en passage (distal to proximal), mobilizing the AVM out of the sylvian fissure (surgeon’s view), and (f) accessing deep feeding arteries along insular cortex (coronal cross-sectional view).
 Microsurgical Anatomy
Microsurgical Anatomy
Brain
Arteries
Veins
 Four Deep AVM Subtypes
Four Deep AVM Subtypes
The Pure Sylvian AVM
The Insular AVM
The Basal Ganglial AVM
The Thalamic AVM
 Resection Strategies for Deep AVMs
Resection Strategies for Deep AVMs
Pure Sylvian AVM Resection
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree