, Peter Giacobbe2 and Andres M. Lozano1
(1)
Division of Neurosurgery, Toronto Western Hospital, University Health Network, University of Toronto, Toronto, Canada
(2)
Department of Psychiatry, Toronto General Hospital, University Health Network, University of Toronto, Toronto, Canada
Abstract
Major Depressive Disorder (MDD) is among the most common psychiatric conditions, and is responsible for substantial human morbidity worldwide. The last two decades have seen significant progress in our understanding of the neural circuits driving MDD, which is now increasingly understood as a disorder of neural circuitry. The success of deep brain stimulation (DBS) as a modulator of circuit dysfunction in motor disorders such as Parkinson’s Disease has generated interest in it’s use in other circuit-based conditions, including MDD. The result has been resurgence in interest in surgery for refractory mood disorders, where advances in functional imaging have helped identify key anatomic targets as critical notes in the circuit. This chapter reviews the history of surgery for major depression, the rationale for focal neuromodulation in the condition, and provides a summary of the clinical experience of DBS in MDD to date.
9.1 Background
Major Depressive Disorder (MDD) is among the most common psychiatric conditions, with a population lifetime prevalence of 14–17 % [1–3]. The costs of MDD are substantial, and represent one of society’s most significant sources of lost wages and productivity [4]. Other costs are more difficult to measure, and relate to the human suffering wrought by an illness that has challenged clinicians for centuries [5]. The last two decades, however, have seen much progress in elucidating the brain circuits driving depressed mood, and offer hope that a better understanding of the illness may lead to new and improved therapeutic options.
MDD is highly heterogeneous. Although sadness is a defining feature, other brain systems such as reward, cognition, and vegetative functions, are involved, suggesting a more complex picture of disease etiology and maintenance. For example, patients with MDD report high degrees of anhedonia, or lack of pleasure with previously pleasurable activities, which implicates dysfunction in reward circuitry. Basal vegetative functions, such as sleep, sexual arousal and appetite, are often disturbed, implicating dysfunction in autonomic and regulatory circuits. Further, symptoms such as rumination, agitation, pathologic crying, and suicidality, all support the notion that MDD is more than merely a ‘deficit’ state [6]. MDD, therefore, cannot be ascribed to a single anatomic structure or circuit, and is likely a manifestation of network wide dysfunction affecting multiple circuits and involving multiple neurotransmitter systems.
The mainstays of treatment for MDD are psychopharmacologic and psychotherapeutic, and are most effective when used in tandem. Medical treatments are aimed at restoring concentrations of key neurotransmitters, most notably serotonin, dopamine and norepinephrine, while psychosocial treatments attempt to identify and correct maladaptive cognitive biases influencing behavior. A large study in nearly 3,000 patients with MDD found that response and remission rates with a single serotonin-reuptake inhibitor (SRI) were 47 and 28 %, respectively [7]. Subsequent studies found that even after continued dose and drug escalations remission rates improved to only 60 % [8, 9]. Such results show that despite optimal medical management, at least one-third of MDD patients remain symptomatic. For these patients neuromodulation options are available [8].
Neuromodulation for MDD can be divided into non-invasive and invasive. The advantages of non-invasive approaches, such as electroconvulsive therapy (ECT) and repetitive transcranial magnetic stimulation (rTMS), are the absence of surgical risk as well as their relatively low-cost and widespread availability. Although effective in some patients, it may be difficult to maintain efficacy in long-term follow-up, and in some instances, such as with ECT, repeated use may be associated with deleterious effects on cognitive functioning [10]. Nevertheless, ECT is highly effective in the management of some types of refractory MDD, and remains the ‘gold-standard’ for neuromodulation in this patient group [8]. Invasive approaches are typically reserved for patients who have failed non-invasive attempts, and provide a permanent or chronic means of adjusting dysfunctional neural circuitry. Next, we review the rationale and experience for invasive neuromodulation in MDD, focusing specifically on the development of DBS.
9.2 Rationale for DBS in Major Depression
Several factors led to the investigation of DBS for MDD. First, was the establishment of DBS as a safe and effective procedure for a range of neurologic, typically motor, diseases [11–14]. As a result, over 100,000 patients world-wide have undergone DBS, most commonly for Parkinson’s Disease, essential tremor, and dystonia [11]. This ability to focally modulate neural circuits motivated the investigation of DBS in other circuit-based disorders, including psychiatric conditions. An additional development has been structural and functional imaging, which have helped identify key nodes in limbic circuitry driving pathologic mood. fMRI and PET helped establish hypothesis-driven models of neural circuit dysfunction, and suggested anatomic targets for DBS procedures. Finally, the existence of a core group of patients, up to a third with MDD, who have no available treatment options, has spurred clinicians to search for novel, safe and effective treatment options (Fig. 9.1).
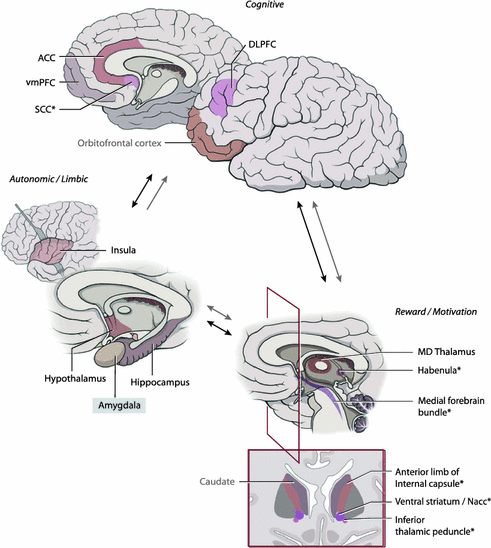

Fig. 9.1
Circuitry of mood and affective regulation. Structures marked with an asterisk have been investigated in DBS trials for major depression. ACC Anterior cingulate cortex, vmPFC ventromedial prefrontal cortex, SCC Subcallosal cingulate cortex, DLPFC dorsolateral prefrontal cortex, MD mediodorsal. Modified with permission from Lozano and Lipsman [11]
9.2.1 Neurosurgery for Depression
Surgery for depression is among the oldest procedures in neurosurgery, with reports of limbic leucotomy , and prefrontal lobotomy, extending back to the early 1940s [15–17]. Early attempts, however, were crude with targeting aided only by surface landmarks, and involving broad disconnections of frontal white matter tracts [16–18]. The introduction of stereotactic techniques, whereby lesions could be generated anywhere in the brain with millimeter-scale accuracy, led to the development of cingulotomy and capsulotomy , which specifically tackled disorders of mood. Cingulotomy involves bilaterally lesioning the anterior cingulate cortex approximately 2 cm posterior to the front of the corpus callosum. The procedure is generally well-tolerated and very safe, with several prospective and retrospective studies describing few adverse effects [19, 20]. A large proportion of patients also derive a significant clinical benefit, with rates of response (defined as a significant reduction in a depression ratings scale and improvements in overall functioning) ranging from 38 to 75 % [20–22]. Capsulotomy involves a lesion in the anterior limb of the internal capsule and is designed to influence fronto-subcortical circuits involved in affective regulation. In one recent paper, 8 patients with refractory MDD underwent bilateral capsulotomy, with 4 of them classified as treatment responders at 2–3 years follow-up [23]. Similar results were reported in a prospective case series of 20 patients, wherein 40 % were in remission and 50 % treatment responders, at a mean follow-up of 7 years [24].
Fifty years of clinical experience with lesions in psychiatric disorders have shown that: (1) Lesions in limbic circuits can be performed accurately and safely; and, (2) Such lesions can effectively influence pathological mood circuits, yielding positive effects in about half of otherwise refractory patients. The disadvantage of lesions , however, remains their permanence. One cannot titrate the clinical effect, ‘escalate’ or ‘reduce’ the dose, or change the location of the lesion once performed. Repeat procedures, particularly with cingulotomy , are routinely done but expose patients to additional surgical risk.
9.2.2 Neurocircuitry of MDD
Several structures have been implicated in circuit models of MDD, including the medial and dorsolateral prefrontal cortex (mPFC; DLPFC), anterior cingulate cortex (ACC), nucleus accumbens /ventral striatum (NAcc/VS), as well as the amygdala. Neuroimaging has been the primer driver of progress in the investigation of mood circuitry, and both structural and functional abnormalities have been found in MDD patients. For example, ACC and hippocampal volumes are both diminished in patients with acute depression, with additional studies finding diffuse gray matter volume reductions. Pre-clinical models have further established a direct link between activity in the nucleus accumbens and both the enjoyment of reward (“liking”) as well as it’s pursuit (“wanting”) [25–27]. Imaging studies have linked activity in reward pathways to both the mPFC and the ventral tegmental area (VTA), a key brainstem dopaminergic center. The ability of VTA to influence both ‘top-down’ cortical centers, via mesocortical pathways, and ‘bottom-up’ regulatory centers, via mesolimbic pathways, has been proposed as a key maintenance system for depressed mood, and anhedonia specifically. For example, dysfunction in both or either mesolimbic or mesocortical systems can result in failure to anticipate or expect a rewarding outcome. Hypoactivation of NAcc in response to otherwise rewarding stimuli has been shown in conditions where reward deficits are well established, such as anorexia nervosa, further linking activity of this structure to affect-laden decisions [28, 29].
Studies performed in healthy subjects and unmedicated MDD patients have shown that activity in the ventral PFC, and subcallosal cingulate (SCC) specifically, is increased in response to sad stimuli in the former, and in the resting state in the latter [30]. This activity is attenuated with medical treatment of depression as well as with DBS in otherwise refractory patients [31, 32]. This has been found in both patients with unipolar depression as well as those with anorexia nervosa with comorbid MDD [33]. Such results have suggested that depression may be linked to a functional ‘decoupling’ of cortical-amygdalar projections, whereby increased activity in both regions leads to a failure of brain homeostatic control over affect [6]. Neurophysiological studies involving recordings directly from neurons are also informing the mechanisms of mood disturbance and the function of key limbic regions. For example, our group has shown using microelectrode recordings from single neurons in the SCC, that neurons in this region fire preferentially to negative pictures compared to neutral or positive ones [34]. Additional work in bipolar depression patients found that SCC neuronal populations undergo synchronization of firing immediately prior to making an emotional decision [35]. These results suggest that this region may be ‘programmed’ to respond to sad and depression-maintaining stimuli.
9.3 Clinical Experience of MDD DBS to Date
Several targets are currently under investigation for DBS in major depression (Table 9.1). These include structures involved in reward (nucleus accumbes/ventral striatum), affective regulation (subcallosal cingulate), and pathways that bridge top-down and bottom-up mood processing (medial forebrain bundle , inferior thalamic peduncle, habenula). Although the global experience with DBS for MDD is growing, all of these trials remain investigational. Below we review the rationale and results to date with the most commonly investigated DBS targets.
Table 9.1
Studies of deep brain stimulation for major depressive disorder, by anatomic target
Study | Number of patients | Outcome |
|---|---|---|
Subcallosal Cingulate | ||
Mayberg et al. [32] | 6 | Follow-up 6 months. 4/6 responders, 2/6 remission as measured by HDRS |
Kennedy et al. [52] | 20 | At last follow-up (3–6 years following implantation, mean = 3.5), response rate = 64.3 % and remission rate = 42.9 % (by HDRS). Considerable improvement in social functioning: 65 % of patients engaged in work-related activity at last follow-up compared to 10 % prior to DBS |
Puigdemont et al. [40] | 8 | Response and remission at 1 year, 62.5 and 50 %, respectively |
Holtzheimer et al. [53] | 17 (10 MDD, 7 with bipolar II) | At one year follow-up, remission and response rate of 36 %. At 2 years, remission rate of 58 % and response rate of 92 %. Remission and response rates based on Hamilton Depression Rating Scale (HDRS). Efficacy similar for MDD and bipolar patients |
Lozano et al. [39] | 20 | At 6 months follow-up, response rate of 48 %; at one-year follow-up, response rate of 29 %. Response measured by HDRS |
Nucleus Accumbens/Ventral Striatum | ||
Schlaepfer et al. [54] | 3 | Double-blind changes to stimulation parameters and assessment. HDRS scores decreased with stimulation and increased with stimulation off |
Malone et al. [44] | 15 | Follow-up from 6–51 months. 8/15 responders and 6/15 in remission at last follow-up measured by Montgomery-Asberg Depression Scale (MADRS) |
Bewernick et al. [41] | 10 | At 12 months, 5/10 had achieved >50 % reduction in HDRS scores (i.e., responders). Antidepressant, antianhedonic, and antianxiety effects observed |
Inferior Thalamic Peduncle | ||
1 | Double-blind assessment protocol following initial period of 8 months with “on” stimulation. No relapse of depressive symptoms with DBS turned off for 12 months. Sustained remission at 24 months with DBS on | |
Habenula | ||
Sartorius et al. [49] | 1 | Remission of MDD following stimulation of the lateral habenula |
Medial Forebrain Bundle | ||
Schlaepfer et al. [47] | 7 | >50 % reduction in depression scores in most patients by day 7 post-op, at 12–33 weeks 6/7 responders, 4/7 in remission |
9.3.1 Subcallosal Cingulate (SCC)
The target with the most experience to date is the subcallosal cingulate cortex (SCC). The SCC is a key node in the affective circuit, receiving inputs from a diverse range of structures including the medial prefrontal cortex, orbitofrontal cortex, anterior cingulate, nucleus accumbens and insula [36, 37]. Additional projections between the SCC and amygdala underscore the relationship, described above, between mood and it’s subcortical regulation by autonomic circuits [6, 36]. Functional imaging studies have shown that the SCC is closely involved in regulating emotions, and in particular negative emotions in both healthy subjects and patients. SCC activity has been linked to the degree of depression, and neurophysiologic studies have confirmed the preferential response of SCC neurons to negative stimuli and decision-making. As a result, the SCC has been proposed as an important node in mood circuitry and the first study of SCC DBS for refractory MDD was performed in 2005. This study included 6 patients and found that at 6-months follow-up, 4 were in remission, and that SCC perfusion, measured using PET , was significantly lower compared to baseline [32]. A larger study, published in 2008 in 20 patients followed to 1-year, found a 50 % response rate [defined as a >50 % reduction in Hamilton Depression Rating (HAMD) Scale scores] [38]. A multicenter study utilizing the same target found a more modest response rate of 29 % at 1-year, which increased to 62 % if treatment response was defined as an improvement in the HAMD by at least 40 % [39]. These results indicated that the majority of patients were either full or partial responders. Similar results were obtained by another group who reported a 50 % response rate with SCC DBS in otherwise refractory patients [40].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







