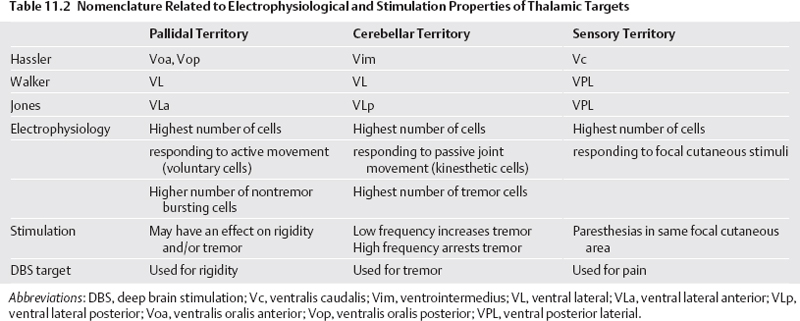
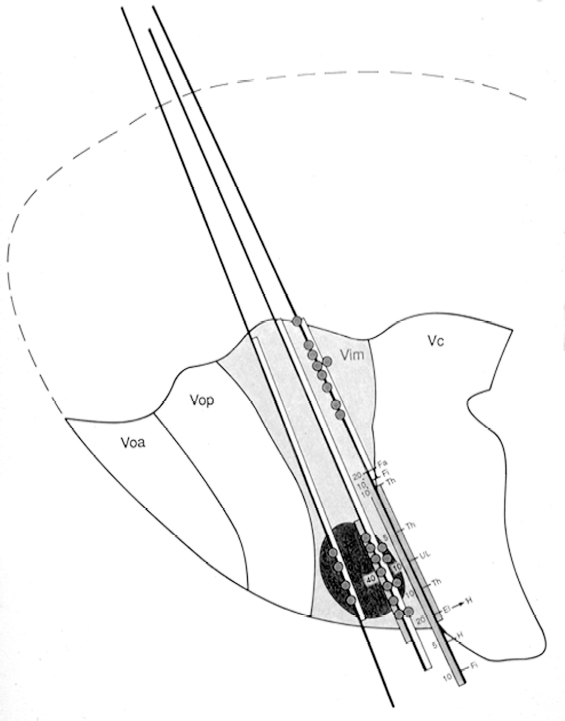
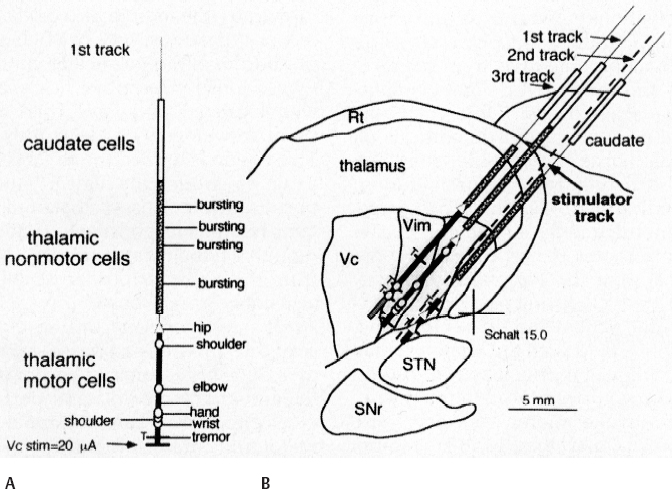
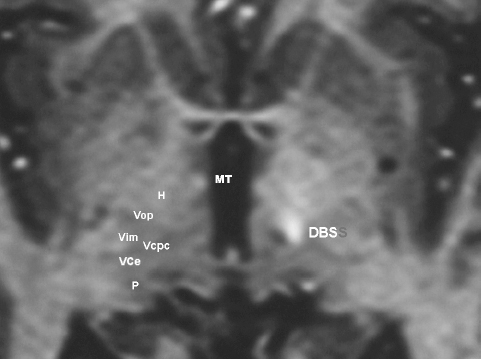
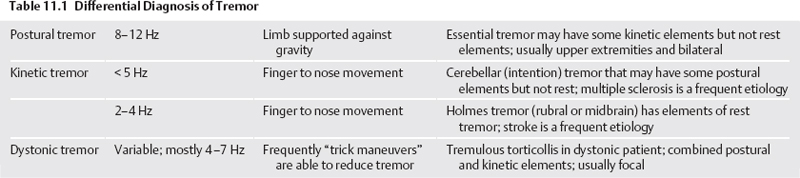
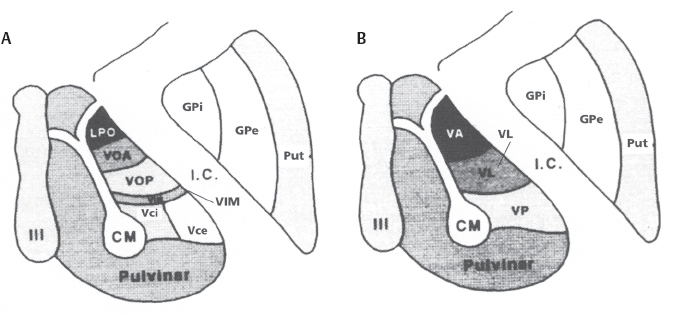
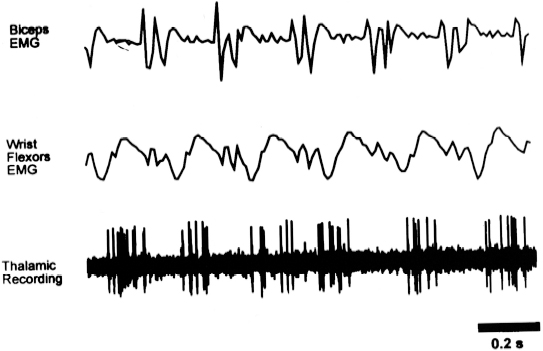
11 Deep Brain Stimulation for Tremor Jorge L. Eller and Kim J. Burchiel Tremor is the most prevalent of the movement disorders and is defined as an involuntary, rhythmic, oscillatory contraction of agonist and antagonist muscles that exhibits a regular frequency. Tremor is further categorized as rest or action (postural, kinetic tremor, task-specific, etc.), depending on when it occurs.1 Resting tremor is defined as tremor occurring in an extremity fully supported against gravity. Postural tremor is defined as tremor occurring when the extremity is maintaining position against gravity. Kinetic (the preferred term over intentional) tremor is defined as tremor occurring with voluntary movement from one point in space to another. Task-specific tremor describes tremor that increases in amplitude during movement directed at a particular goal (e.g., writing tremor). Moreover, tremor can be further characterized objectively with electromyography, accelerometry, and Fourier analysis.2,3 The differential diagnosis of tremor divides it into specific clinical entities, the most common being essential tremor (ET), parkinsonian tremor, cerebellar outflow tremor, Holmes tremor (also called rubral tremor), and dystonic tremor (Table 11.1). These can be differentiated based upon features in the history and physical exam as well as electrophysiological characteristics.4 Epidemiological studies of ET confirm that it is the most common movement disorder. A 45-year retrospective analysis of medical records from Rochester, Minnesota, revealed the incidence and prevalence of ET were 23.7 and 305.6 per 100,000 cases, respectively. Mean age of diagnosis was 58 years, with men and women being equally affected. The investigators noted that age-specific incidence increased after 49 years of age and peaked in the 80s.5–7 Although a gene variant (HS1-BP3) on chromosome 2 is associated with familiar ET, the pathophysiology of ET remains unknown. No obvious histopathologic abnormalities are associated with ET; therefore, no animal models are available that conclusively replicate human ET. An aberrant central oscillator in the inferior olive is hypothesized as one possible cause of ET. The rationale is that the olivocerebellar oscillations are transmitted via the cerebellothalamocortical pathway, thus encompassing the cerebellum, thalamus, and brainstem areas that are known to be involved in ET.8,9 The early days of functional neurosurgery involved destruction of parts of the motor system.10–12 Surgery to treat movement disorders became popular following Spiegel and Wycis’s 1947 development of the first stereotactic apparatus that enabled minimally invasive surgery on deep nuclei in the human brain.12 Further refinement eventually led to the determination that Hassler’s ventrointermedius (Vim) nucleus of the thalamus is the optimal surgical target (Fig. 11.1). Regardless of tremor etiology, Vim is the preferred target of surgery to treat tremor except dystonic tremor (see Chapter 12) and of course psychogenic tremor. The Vim is the major termination of cerebellar efferents of the cerebellothalamocortical pathway (Fig. 11.2) and projects to the motor cortex.14 This area contains the greatest concentration of tremor cells or neurons that fire in bursts synchronous with the patient’s tremor (Fig. 11.3). In addition to the anatomical connection revealed in primate tracing studies, positron emission tomography (PET) has demonstrated an increase in blood flow to the ipsilateral motor cortex with stimulation of the thalamic Vim, suggesting a functional connection between these structures.15 The surgical repertoire available to treat tremor includes either ablation of the contralateral thalamic Vim by means of radiofrequency lesion (thalamotomy) or placement of a deep brain stimulation (DBS) electrode in the contralateral thalamic Vim. In 1991, Benabid et al published an article that described tremor suppression with chronic high-frequency stimulation of the thalamic Vim nucleus.16 Although it had been known since the 1950s that high-frequency stimulation of the Vim nucleus could arrest tremor acutely, this introduced the first practical technique for chronic brain stimulation as an alternative to lesioning (thalamotomy) in the treatment of tremor. Indications for both procedures are similar. Basically, patients must have tremor that is refractory to medical therapy and represents the predominant manifestation of the disorder. The ideal candidates for thalamic surgery are patients with ET and those with tremor-predominant Parkinson disease (PD), although surgery may also be considered for tremor secondary to multiple sclerosis, stroke, or trauma. The remainder of this chapter describes our surgical technique and experience with placement of DBS electrodes to treat tremor. Fig. 11.1 Terminology of the thalamic nuclei. (A) Hassler classification system. (B) Anglo-American classification system. LPO, lateral polaris; VOA, ventral oral anterior; VOP, ventral oral posterior; VIM, ventral intermediate; Vci, ventral caudal internal; Vce, ventral caudal external; CM, centrum medianum; VA, ventral anterior; VL, ventral lateral; VP, ventral posterior; GPi, internal segment of the globus pallidus; GPe, external segment of the globus pallidus; put, putamen; I.C., intercommissural region; III, third ventricle. (From Burchiel KJ. Thalamotomy for movement disorders. Neurosurg Clin N Am 1995;6:55–71.) Fig. 11.2 Thalamic neuroanatomy relevant to neurosurgery. The major inputs to the sensory and motor thalamus are illustrated using Hassler’s nomenclature and alternative nomenclature. The ventral lateral (VL) nucleus is roughly the equivalent of Hassler’s nucleus ventralis oralis anterior (Voa), nucleus ventrali oralis posterior (Vop), and ventral intermediate (Vim). Vim receives both vestibulothalamic and cerebellothalamic afferents. Vop may also receive cerebellothalamic afferents. Both Vop and Voa receive pallidothalamic fibers from the medial globus pallidus interna (Gpi). Fig. 11.3 Tremor cells recorded from the VIM (bottom). Note the correlation between the neuronal discharges from the tremor cells and the electromyogram. These discharges stop when the tremor ceases. (Modified from Bakay RAE, Vitek JL, Delong MR. Thalamotomy for tremor. In: Rengechary SS, Wilkins RR, eds. Neurosurgical Operative Atlas. Vol 2. Park Ridge, IL: American Association of Neurological Surgeons; 1992:299–312.) The surgical implantation of DBS electrodes involves sequential steps, and optimal results require careful performance. Placement of a stereotactic frame is the first step and sets the stage for magnetic resonance imaging (MRI)-guided stereotactic localization. At our institution, we use the Leksell Stereotactic System (Elekta Inc., Norcross, GA, www.elekta.com/healthcareus.nsf). To obtain images that easily correlate with standard brain neuroanatomical atlases, this frame requires placement orthogonal to the anatomical planes of the brain. The frame has ear bars to prevent lateral tilt or rotation. The anteroposterior axis of the frame should be placed parallel to a line connecting the glabela to the inion, which places the frame parallel to the anterior commissure–posterior commissure (AC–PC) line.56 Anatomical targeting of the Vim is the next step and employs a combination of indirect and direct targeting. Indirect targeting is based upon fixed distances from the midpoint of a line connecting the AC to the PC to the Vim nucleus. The locations of the AC and PC can be determined by MRI, CT, or ventriculography. MRI is slightly less accurate than CT, with average errors of ~2 mm due to artifacts related to inhomogeneities in the magnetic field. In an attempt to decrease such errors, imaging fusion, or overlapping of CT and MRI data, is often used.57–59 Targeting of the Vim nucleus is then accomplished with the use of computer programs such as the FrameLink Stereotactic Linking System software (Medtronic Neuro Navigation, Louisville, CO, www.MedtronicNavigation.com) that relate a standard atlas10 to the patient’s radiological anatomy, as measured by CT or MRI.60 In this manner the radiological location of the Vim nucleus is converted to Cartesian coordinates on the Leksell frame. In an attempt to compensate for individual variations in nuclear location, direct targeting is based upon direct visualization of nuclear boundaries in an MRI T2 fast spin echo (FSE) sequence. Unfortunately, it is not always possible to clearly visualize target borders in MRI sequences. The patient is brought to the operating room and positioned supine, with the head slightly elevated. The Leksell frame is fixated to the Mayfield headholder with an adapter. Standard prepping and draping are performed and local anesthetic injected. A single bifrontal semicurve C-shaped incision is performed, anterior to the coronal suture. Burr holes are placed anterior to the coronal suture and 2.5 cm from the midline on either side. Dura and pia are coagulated and opened sharply. The Leksell arc is positioned for a standard trajectory of 60 degrees from the AC–PC line. Due to limitations inherent to mechanical properties of the stereotactic frame and the imaging technique employed, anatomical targeting alone is insufficient for optimal electrode placement. Standard stereotactic frame system precision has been measured to be approximately within 1.5 mm (95% confidence interval).61 Furthermore, MRI distortion effects, imperfect visualization of the anatomical target, potential brain shift, and variability of physiological function within any specific anatomical target further decrease the accuracy of anatomical targeting.62 Therefore, physiological methods for localization are important to confirm and if necessary adjust the final placement of the DBS lead. Two physiological methods to refine anatomical targeting are microelectrode recording (MER) and macrostimulation. MER isolates single neuronal action potentials using platinumiridium or tungsten microelectrodes with an impedance greater than 500 KΩ.63,64 The microelectrode is attached to a microdrive and mounted on the stereotactic frame. The microelectrode tip is retracted into a protective cylindrical sheath and inserted to a standard depth, from which the microdrive is used to drive the microelectrode toward the target,65 and the signal from the microelectrode is amplified and filtered. Neuronal discharges are viewed on an oscilloscope and sound is heard through an audio monitor. Distinguishing characteristic patterns of spontaneous discharge within the nuclei of the basal ganglia are then used for localization. Macrostimulation is employed to identify the target area in the Vim nucleus by a high-frequency stimulation-evoked decrease in tremor. Similarly macrostimulation can be used to identify the internal capsule (by stimulation-evoked contractions of skeletal muscle), and the thalamic sensory ventralis caudalis (Vc) nucleus (by stimulation-evoked paresthesias). At our institution, once the target is defined bilaterally using anatomical and physiological techniques, we proceed to implant the DBS leads. There are two US Food and Drug Administration (FDA)-approved lead models (Medtronic Minneapolis, MN, www.medtronic.com/physician/activa/techmanuals.html). Both are quadripolar with four platinumiridium contacts at the distal end. Each contact is 1.5 mm in length and separated by wide (1.5 mm) spacing (model #3387) or narrow (0.5 mm) spacing (model #3389). The lead is implanted along the desired MER trajectory with the second contact from the tip (#1 contact) positioned at the target. The lead is then secured in place using the Stimloc ring and cap lead anchor system (Medtronic, Inc., Minneapolis, MN). Intraoperative lateral fluoroscopy is used to confirm that the DBS lead remains in position after being detached from the stereotactic frame and secured in the Stimloc device. The procedure is repeated for the DBS lead on the other side. The DBS leads are then tunneled under the subgaleal tissue, and the surgical wound is closed with special care to avoid damaging the leads. The stereotactic Leksell frame is then removed. To finalize the procedure the patient is placed under general anesthesia, positioned supine with the head turned away from the side of the implant. An internal pulse generator (IPG) is usually placed in a subcutaneous pocket below the clavicle. Therefore, the bifrontal incision, as well as the ipsilateral retroauricular, cervical, and subclavicular areas, are prepped and draped in sterile fashion. To accommodate the IPG an incision is performed 2.5 cm below the clavicle, and a subcutaneous pocket is created over the pectoralis fascia. A second incision is created 2.5 cm posterior to the ipsilateral ear for placement of the connectors between the distal ends of the DBS leads and the extension cables that will connect the DBS leads to the IPG. Care is taken to assure that these connectors will sit over the periosteum, in a sub-galeal pocket created to accommodate them, and not in the neck, where excessive movement may cause lead fracture. The extension cables are tunneled from the subclavicular pocket to the retroauricular incision. In a similar fashion, the distal ends of the DBS leads are retrieved and tunneled from the bifrontal incision (which is reopened carefully so as not to damage the leads) to the retroauricular incision. The DBS leads are connected to the extension cables using a standard four-screw connector, which is protected by a Silastic (Dow Corning, Midland, MI) sheath and placed in the subgaleal pocket mentioned earlier. The distal ends of the extension cables are then connected to the IPG. All wounds are irrigated with antibiotic irrigation and closed in layers. The DBS IPG is programmed using an external programming device. Adjustable stimulation variables include amplitude, frequency, pulse width, and choice of active contacts (see Chapter 13).
Stereotactic Frame Placement
Anatomical Targeting
Surgical Exposure and Trajectory
Physiological Targeting
Deep Brain Stimulation Lead Placement and Anchoring
Internal Pulse Generator Placement
Deep Brain Stimulation Programming
Neupsy Key
Fastest Neupsy Insight Engine