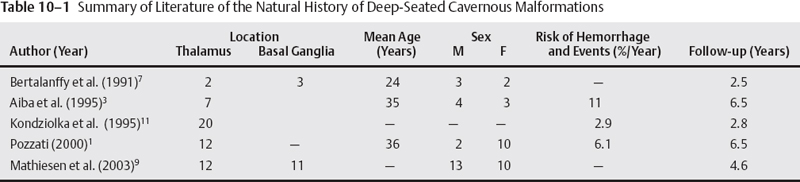
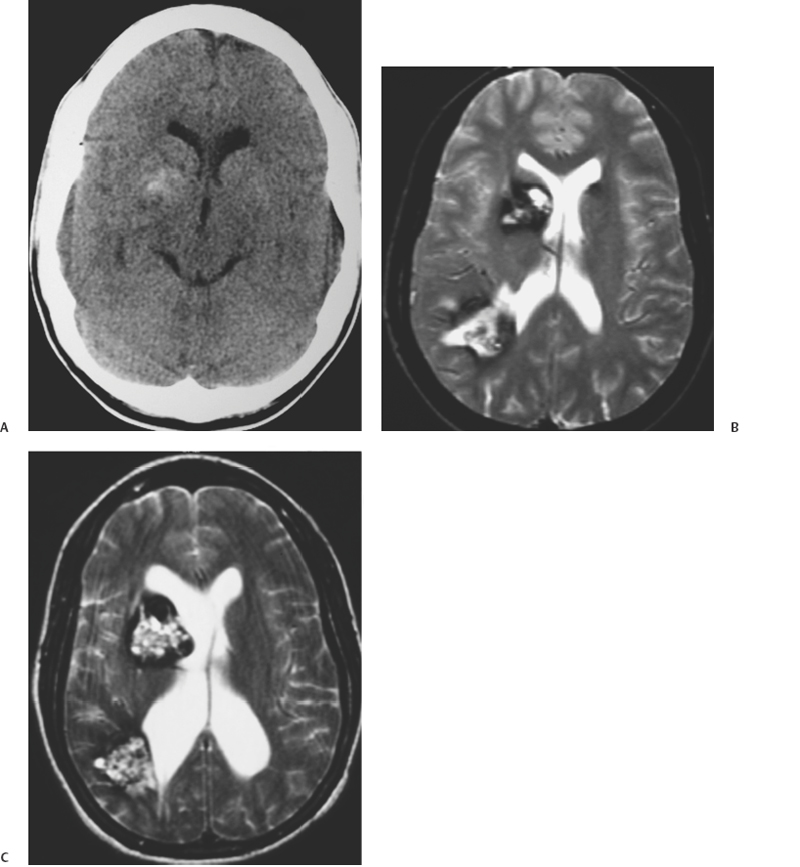
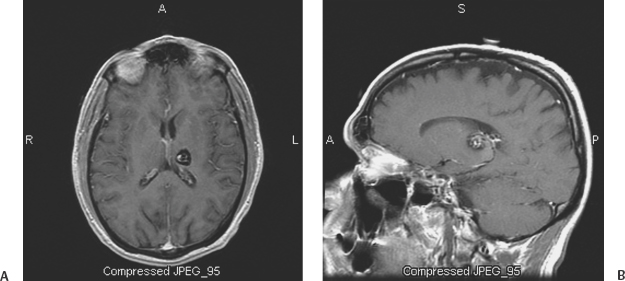
10 Uygur Er, Robert F. Spetzler, Andrea Cardia and Giuseppe Lanzino Deep-seated cavernous malformations (CMs) constitute a subgroup of lesions located in the basal ganglia, thalamus, periventricular area, insular-subinsular region, ventricular system, and brain stem. These lesions pose significant challenges because of the critically important neurologic functions associated with these small and vulnerable cerebral regions. This chapter considers only CMs located in the thalamus and basal ganglia because CMs located in the ventricles, insular-subinsular region, and the brain stem are treated elsewhere in this book. Analysis of the published literature in relation to CMs of the thalamus and basal ganglia is difficult as only a few reports have concentrated on these particular locations,1 and many series have considered these deep-seated lesions in conjunction with brain-stem CMs. Similar to CMs located in other locations throughout the central nervous system (CNS), thalamic and basal ganglia CMs have been considered to occur infrequently, and little was known about their clinical significance. The widespread introduction of magnetic resonance imaging (MRI) has dramatically increased detection of these lesions.2–5 The distribution of CMs is representative of the distribution of the neural tissue along the cranio-spinal axis.2 However, CMs of the thalamus and basal ganglia are reported to range between 6.9% and 21.7% of all CMs in the literature.1,3,6–8 This great variability probably reflects referral biases inherent to these series, which are primarily surgical series from prominent tertiary referral centers. A recent report has calculated the incidence of the deep-seated CMs to be ~3.2/1,000,000 per year and 0.8/1,000,000 per year when symptomatic and incidental lesions are considered, respectively.9 Some series suggest a significant difference in the sex predilection of thalamic and basal ganglia CMs6 with a female preponderance observed especially among symptomatic patients.1,3 Other authors, however, have observed equal distribution between the two sexes.7 The age range at diagnosis varies between 5 and 54 years with most lesions diagnosed in the third and fourth decades of life1,7 (Table 10-1). The clinical presentation of deep-seated CMs is quite different than lesions located in other portions of the CNS. Patients with symptomatic lesions often suffer from the sudden onset of various constellations of symptoms and signs. The onset of symptoms generally occurs before the third decade of life. Symptoms and signs at presentation are closely related to the location of the lesion. Patients with thalamic CMs frequently present with acute motor and/or sensory symptoms and signs with or without headache. Lesions located in the medial thalamus or those lesions with extension toward the third ventricle can cause hydrocephalus1,7,9 (Fig. 10-1). Hydrocephalus as presenting symptom can occur in up to 15% of thalamic CMs.9 Of course, seizures as presenting symptom are less frequently observed in deep-seated lesions when compared with more superficial ones.1,8 Lesions located in the basal ganglia can also cause hemichorea, focal dystonia, and other basal ganglia-related symptoms. Although the likelihood of hemorrhage appears approximately equal among CMs, regardless of their location,2 CMs in critically eloquent areas such as the thalamus become symptomatic even with minimal volumetric changes of the lesion and give a false impression of higher hemorrhage rates when compared with CMs located in less eloquent areas.10 The incidence of hemorrhage also varies according to the criteria used in defining it (clinical, radiologic, or both). The fact that different criteria have been used to define hemorrhagic risk in the literature makes it difficult to compare different series. This also explains the great variability in the rate of first hemorrhage. It is calculated that the overall risk of first hemorrhage from a thalamic or basal ganglia CM ranges between 0.7% and 5% per year. In patients with prior history of hemorrhage, the reported annual subsequent bleeding rate varies between 4.5% and 30%.10,11 There is no significant difference in the annual bleeding rate between familial and sporadic cases. History of prior hemorrhage is the most important risk factor for subsequent hemorrhage. The location of the CM (thalamus or basal ganglia) does not correlate with subsequent hemorrhage risk.11 Similar to CMs located in other areas, deep-seated supratentorial CMs can increase in size over time.5 Multiple lesions are observed in up to 2.5% of patients with deep-seated CMs.7,9 Figure 10-1 (A) Computerized tomography of the brain done in 1990 shows multiple calcified lesions. The patient, at that time 20 years old, was followed with serial MRI studies. (B) Axial, T2-weighted MRI done in 1993 reveals the presence of a right anterior thalamic lesion in addition to other abnormalities with the characteristic appearance of CMs. The patient continued to remain asymptomatic until 1998 when (C) she developed signs and symptoms of hydrocephalus. She has been treated with bilateral ventriculoperitoneal shunts and has remained symptom-free since. Further MRI studies have shown no evolution of the multiple CMs. Surgical treatment of deep-seated CMs is indicated in symptomatic patients who have suffered prior hemorrhage. Radical surgical excision of the lesion eliminates the risk of subsequent bleeding and may result in improvement of symptoms in patients suffering from mass effect.9,12 Similar to CMs located in other highly eloquent areas such as the brain stem and the spinal cord, the decision on whether or not to surgically excise these lesions depends on their location in relation to the pial or ependymal surface. Lesions that abut the ventricular or pial surface can be safely excised without the need of traversing eloquent structures. However, in the case of basal ganglia and thalamic CMs, recent surgical experience indicates that surgical excision can be successfully performed also in the case of symptomatic lesions located deep within the brain surface without causing additional morbidity.9 In patients who present with symptomatic hydrocephalus without a history or neuroradiologic documentation of hemorrhage, treatment of hydrocephalus alone is a reasonable option (Fig. 10-1). The role of alternative treatment modalities (i.e., Gamma Knife) in surgically inaccessible lesions is controversial4,13–15 and is discussed extensively elsewhere in this book. There is no consensus about the timing of surgery in patients who have suffered a hemorrhage. Some authors prefer to wait 4 weeks to permit neurologic recovery and stabilization of the lesion.12 Others suggest operating in the subacute period because the fresh hemorrhage makes recognition of the cleavage plane and dissection of the lesion easier. In addition, it has been observed that hematoma organization and formation of an adherent gliotic plane around the lesion over time can make the dissection more difficult, and it is associated with an increased risk of postoperative transient and permanent neurologic deficits.9 It is our preference to perform surgery whenever possible in the subacute phase (within 2 to 3 weeks) after a symptomatic episode. A shorter time interval between a symptomatic hemorrhage and surgery also decreases the risk of further deterioration from additional hemorrhages, which seems to be higher in the first 18 to 24 months after an initial episode.16 Surgical planning depends on the specific location of the lesion and its position in relation to the ependymal or pial surface. Assessment of the resectability of these lesions and their relationship to the pial or ependymal surface should be based on the T1 sequence of the MRI (Fig. 10-2). On the T2-weighted images, a “blooming” artifact from the hemosiderin-stained surrounding tissue may give a false impression of superficiality. Intraoperative frameless localization has been in our and other authors’12 experience an invaluable tool in facilitating the intraoperative localization and resection of such lesions. Because of their central location, usually there are no problems related to inaccuracy of the frameless system secondary to intraoperative brain shift. This is true even in case of lesions approached through a transventricular approach during which significant amounts of cerebrospinal fluids are drained. Deep-seated lesions of specific sites require different surgical approaches. Schematically, CMs of the thalamus and basal ganglia can be divided in medial and lateral lesions. Most thalamic CMs can be reached through an ipsilateral or contralateral interhemispheric transcallosal approach.9,12,17–19 We prefer a contralateral approach in which the patient is placed supine with the head positioned with the sagittal suture parallel to the floor and elevated 45 degrees (Figs. 10-3A, B). Too much neck torsion should be avoided to prevent venous return obstruction. The side bearing the lesion is placed up. By approaching the lesion from the contralateral side, a more direct view of the CM is obtained when compared with the ipsilateral interhemispheric approach (Fig. 10-4). In addition, gravity drops the ipsilateral (to the approach) hemisphere so minimal or no retraction is required (Fig. 10-4A). The contralateral approach also affords better visualization of the most lateral aspect of the lesion, which would be hard to visualize from an ipsilateral approach without significant retraction of the ipsilateral hemisphere (Fig. 10-4A). We prefer a frontoparietal U-shaped skin incision that extends across the midline (Fig. 10-3B). The craniotomy is performed with two-thirds of the bone flap anterior and one-third posterior to the coronal suture (Fig. 10-3B). The craniotomy is taken across the superior sagittal sinus, and the dura is opened in a curvilinear fashion with the dura flap based on the superior sagittal sinus. The dura is then tented to the contralateral side. This maneuver elevates the lateral margin of the sinus improving the surgeon’s view into the interhemispheric fissure. The angle of the approach can be tailored to the position of the specific lesion (Fig. 10-5). Through this approach, lesions localized in the posterior thalamus can also be successfully resected. The corpus callosum is then identified and a small callosotomy done with microsurgical dissection following the indications of the frameless stereotactic system (Figs. 10-6A, B). In some cases, after entering the ventricle, the lesion may not be visible on the surface of the thalamus (Fig. 10-7A). In such cases, the incision on the thalamic surface is made in correspondence with the thinnest layer of eloquent parenchyma covering the CM as indicated by the frameless system (Fig. 10-7B).
Deep-Seated Cerebral Cavernous Malformations
 Epidemiology
Epidemiology
 Natural History and Clinical Presentation
Natural History and Clinical Presentation
 Surgical Treatment
Surgical Treatment
Indications and Surgical Technique
Deep-Seated Cerebral Cavernous Malformations
Only gold members can continue reading. Log In or Register to continue

Full access? Get Clinical Tree



