, Jean Paul G. Vonsattel2, Helmut Heinsen3, 4 and Horst-Werner Korf5
(1)
Dr. Senckenbergisches Chronomedizinisches Institut, Goethe University Frankfurt, Frankfurt, Germany
(2)
Medical Center Neurological Institute, Columbia University, New York, NY, USA
(3)
Division Psychiatic Clinic Morphological Brain Research Unit, Julius Maximilians University Würzburg, Würzburg, Germany
(4)
University of Sao Paulo Medical School, Sao Paulo, Brazil
(5)
Dr. Senckenbergisches Chronomedizinisches Institut, Goethe University, Frankfurt, Frankfurt, Germany
4.1 The Thalamus in Huntington’s Disease (HD)
Although early neuropathological studies reported degenerative features in subcortical brain regions of HD patients (e.g., pallidum, subthalamic nucleus, substantia nigra, claustrum, amygdala) including the thalamus (e.g., global atrophy and volume loss of the thalamus; thalamic astrogliosis; reduced density of microneurons in its cerebellar territory, the ventrolateral nucleus; astrogliosis in the centromedian and parafascicular nuclei; shrinkage of the centromedian nucleus and its nerve cells) (see Chap. 1) (Borrell-Pagès et al. 2006; Dom et al. 1976; Finkbeiner and Mitra 2008; Lange and Aulich 1986; Lange et al. 1976; McCaughey 1961; McLardy 1948; Pfeiffer 1913; Terplan 1924; Vonsattel 2008; Vonsattel and DiFiglia 1998; Vonsattel et al. 1985; Walker 2007a, b), the involvement of the thalamus and the extent of its degeneration in HD have been neglected in more recent investigations. Therefore, the affection of the thalamic nuclei is currently not among the established degenerative features of HD.
According to current pathoanatomical concepts, the typical distribution pattern of brain neurodegeneration in HD is thought to be the result of an anterograde, retrograde, or transneuronal and topographically highly ordered spread of the underlying pathological process throughout the brain via interconnecting brain fiber tracts. Spreading of this process may involve a prion–like mechanism (Brundin et al. 2010; Costanzo and Zurzolo 2013; Goedert et al. 2010; Jucker and Walker 2011; Labbadia and Morimoto 2013; McLardy 1948; Millecamps and Julien 2013; Norrby 2011; Renner and Melki 2014). In view of this possibly targeted spread of the disease process along anatomical pathways, it appeared conceivable that brain regions possessing intimate anatomical connections with the affected regions of the cerebral cortex (e.g., prefrontal cortex) and/or the neostriatum may also be among the targets of the neurodegenerative process of HD (Heinsen et al. 1996, 1999; Heinsen and Rüb 1997). In view of the severe degeneration of the neostriatum and the layer-specific neuronal loss in the prefrontal cortex, it appeared plausible that the thalamic centromedian–parafascicular complex (CM–PF) and mediodorsal nucleus (MD) which are interconnected with these cortical and subcortical predilection sites of HD-associated neurodegeneration may also be among the primary or secondary targets of the disease process of HD. Indeed, these theoretical assumptions have been confirmed by recent investigations that applied modern stereological methods (i.e., volume assessments according to the Cavalieri’s principle; cell counts with the optical disector) on thick frontal serial tissue sections through the thalamus stained with gallocyanin for neuronal Nissl material and demonstrated the consistent neurodegeneration of both thalamic nuclei in HD patients (Heinsen and Heinsen 1991; Heinsen et al. 1996, 1999).
4.2 Functional Neuroanatomy of the Centromedian-Parafascicular Complex (CM-PF) of the Human Thalamus
The centromedian–parafascicular complex (CM–PF) (Fig. 4.1) is a collection of neurons that belong to the caudal intralaminar nuclear group, which is located in the posterior part of the thalamus. The parafascicular nucleus (PF) (Fig. 4.1) occurs in all mammals and, in contrast to the other thalamic nuclei (e.g., the ventrolateral nucleus), is well conserved during the phylogeny of the primate brain. The centromedian nucleus (CM) (Fig. 4.1), however, appears later in the evolution, is enlarged in parallel with the disproportionally increase of the putamen, becomes increasingly prominent in primates, and reaches its maximal development in humans (Armstrong 1990; Fenelon et al. 1991; Rapoport 1990; Sadikot and Rymar 2009; Sadikot et al. 1992b). The CM and PF are the source of the main thalamic afferents to the striatum (Fig. 4.2) and together innervate most portions of the striatum in primates in a topographical order. The CM represents a nodal point in the closed reciprocal neostriatal-pallido-centromedian–neostriatal loop of the basal ganglia, is the most prominent source of thalamostriatal projections, and is intricately linked to both components of the neostriatum (i.e., putamen and caudate nucleus) by efferent projections. Via these striatofugal projections, the CM is involved in the regulation of the activity of the neostriatal output neurons. The CM receives its main afferents from the internal segment of the pallidum via the lenticular ansa and the lenticular and thalamic fascicles. It is therefore included into the motor basal ganglia-thalamocortical feedback loop, is mainly associated with the sensorimotor territory of the striatum (Fig. 2.9), and can serve sensorimotor integrative functions and processing of motor information to the neostriatum (Fenelon et al. 1991; François et al. 1991; Groenewegen 2003; Haber and Calzavara 2009; Heinsen et al. 1996; Nakano et al. 2000; Parent 1990; Parent and Hazrati 1995; Sadikot and Rymar 2009; Sadikot et al. 1992a; Sadikot et al. 1992b; Walker 1982). The PF projects mainly to the associative territory of the striatum (Figs. 2.9 and 4.2) and less abundantly to its limbic territory (e.g., accumbens nucleus) (Fig. 2.9). In addition, the PF is reciprocally interconnected with the oculomotor frontal and supplementary eye fields of the cerebral cortex. Owing to its anatomical interconnectivities and its relation with the associative and limbic territories of the striatum and the oculomotor network, the PF is involved in associative-limbic brain functions (i.e., cognitive and emotional processes), as well as oculomotor functions (Fenelon et al. 1991; Heinsen et al. 1996; Huerta et al. 1986; Künzle and Akert 1977; Nakano et al. 2000; Parent 1990; Parent and Hazrati 1995; Sadikot and Rymar 2009; Sadikot et al. 1992a; Sadikot et al. 1992b; Stanton et al. 1988).
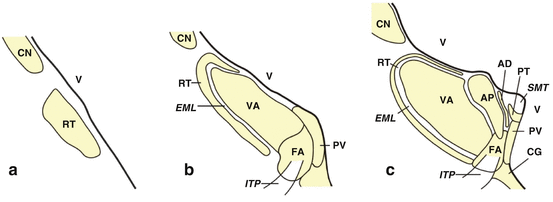
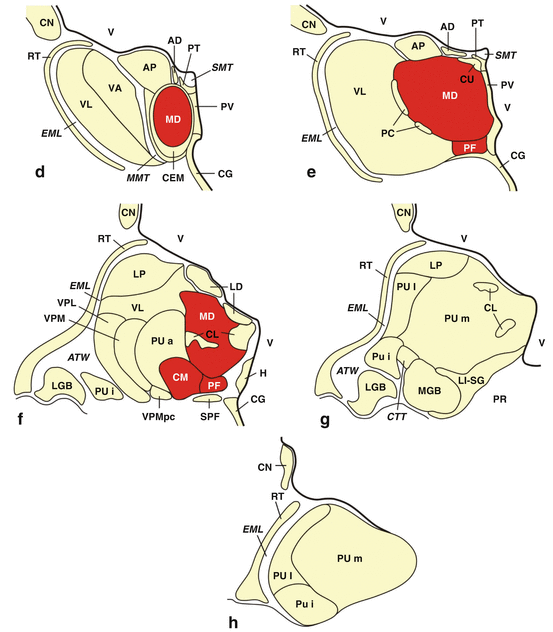
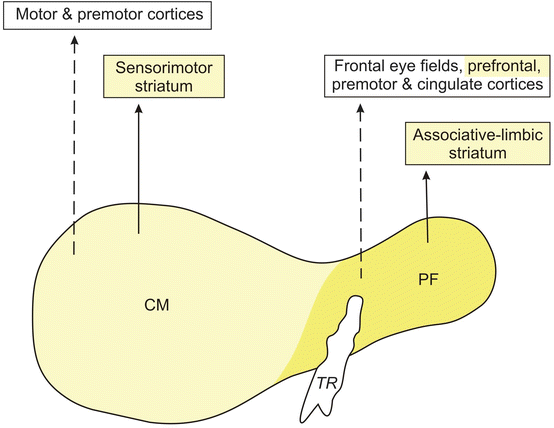


Fig. 4.1
The human thalamus. Schematized frontal sections cut perpendicularly to Forel’s intercommissural axis showing the rostrocaudal sequence of the nuclei of the human thalamus. (a) Rostral pole of the human thalamus made up by the reticular nucleus (RT). (b) Frontal section through the rostral pole of the human thalamus with the lateral portion of the reticular (RT), ventral anterior (VA), fasciculosus (FA), and paraventricular nuclei (PV). (c) The human thalamus with the reticular (RT), ventral anterior (VA), fasciculosus (FA), paraventricular (PV), parataenial (PT), anterodorsal (AD), and anteroprincipal nuclei (AP). (d) The rostral thalamus at the level of the mammillothalamic tract (MMT) showing the reticular (RT), ventral lateral (VL), ventral anterior (VA), central medial (CEM), mediodorsal (MD), paraventricular (PV), parataenial (PT), anterodorsal (AD), and anteroprincipal nuclei (AP). (e) The mid-level of the human thalamus comprises the reticular (RT), ventral lateral (VL), mediodorsal (MD), paracentral (PC), parafascicular (PF), paraventricular (PV), cucullar (CU), parataenial (PT), anterodorsal (AD), and anteroprincipal nuclei (AP). (f) Frontal section through the caudal thalamus at the level of the habenular nuclei (H): reticular nucleus (RT), lateral geniculate body (LGB), inferior nucleus of the pulvinar (PU i), lateral posterior nucleus (LP), ventral lateral nucleus (VL), ventral posterior lateral (VPL) and ventral posterior medial nuclei (VPM), anterior nucleus of the pulvinar (PUa), ventral posterior medial nucleus, parvocellular part (VPMpc), centromedian (CM), parafascicular (PF), subparafascicular (SPF), central lateral (CL), mediodorsal (MD), and laterodorsal nuclei (LD). (g) The human thalamus at the level of the pretectum with the reticular (RT) and lateral posterior nuclei (LP), the lateral (PU l) and inferior nuclei of the pulvinar (PU i), the lateral (LGB) and medial geniculate bodies (MGB), the medial nucleus of the pulvinar (PU m), the central lateral nucleus (CL), as well as the limitans-suprageniculate complex (LI–SG). (h) Caudal pole of the human thalamus with the reticular nucleus (RT) and the lateral (PU l), inferior (Pu i), and medial nuclei (PU m) of the pulvinar. The nuclei investigated by means of modern stereological methods in HD (i.e., MD, CM, and PF) are colored in red (Modified according to Rüb et al. (2003b), (Figure 1, page 2260); with kind permission from Oxford University Press). Abbreviations: AD anterodorsal nucleus, AP anteroprincipal nucleus, ATW triangular area of Wernicke, CEM central medial nucleus, CG central gray, CL central lateral nucleus, CM centromedian nucleus, CN caudate nucleus, CTT corticotectal tract, CU cucullar nucleus, EML external medullary lamina, FA fasciculosus nucleus, H habenular nuclei, ITP inferior thalamic peduncle, LD laterodorsal nucleus, LGB lateral geniculate body, LI-SG limitans-suprageniculate complex, LP lateral posterior nucleus, MD mediodorsal nucleus, MGB medial geniculate body, MMT mammillothalamic tract (Vicq d’Azyr), PC paracentral nucleus, PF parafascicular nucleus, PR pretectum, PT parataenial nucleus, PUa anterior nucleus of the pulvinar, PU i inferior nucleus of the pulvinar, PU l lateral nucleus of the pulvinar, PU m medial nucleus of the pulvinar, PV paraventricular nucleus, RT reticular nucleus, SMT stria medullaris thalami, SPF subparafascicular nucleus, V ventricle, VA ventral anterior nucleus, VL ventral lateral nucleus, VPL ventral posterior lateral nucleus, VPM ventral posterior medial nucleus, VPMpc ventral posterior medial nucleus of the thalamus, parvocellular part

Fig. 4.2
The major projections of the human thalamic centromedian-parafascicular complex (CM-PF) to the cerebral cortex and striatum. Diagram of a frontal section through the human thalamic centromedian (CM, light yellow) and parafascicular (PF, yellow) nuclei with their main efferent outputs to the cerebral cortex (dashed lines) and the “sensorimotor,” “associative,” and “limbic” territories of the striatum (unbroken lines). All cortical and subcortical sites depicted in this diagram that have been convincingly shown to sustain neuronal loss in Huntington’s disease (HD) are colored either in yellow or light yellow (Modified according to Sadikot et al. (1992b), (Figure 12, page 156); with kind permission from John Wiley and Sons). Abbreviations: CM centromedian nucleus of the thalamus, PF parafascicular nucleus of the thalamus, TR tractus retroflexus, habenulo-interpeduncular tract
The CM–PF of the human thalamus is embedded into an extension of the internal medullary lamina and represents the main component of the caudal intralaminar group (Fig. 4.1). The CM-PF is rostrally and dorsally bordered by the large, associative thalamic MD and laterally by the somatosensory ventroposterior medial and the gustatory parvocellular portion of the ventroposterior medial thalamic nuclei. Separated from it by a small medullary lamina, the subparafascicular nucleus lies ventrally to the CM-PF and is integrated into the limbic circuits of the human brain. Medially, the CM-PF abuts on the central gray and merges dorsally with the pulvinar and the limitans-suprageniculate complex, which marks the border between the thalamus and the midbrain pretectum (Figs. 4.1 and 4.4). Although many authors recognized two subnuclei or suggested a tripartite architectonic subdivision of the human CM-PF complex, all investigators of the normal anatomy of this thalamic complex agree that the lateral boundaries of the medially situated PF are complex and interdigitate without sharp transitions with the medial parts of the CM (Fig. 4.4) (Hassler 1982a; Heinsen et al. 1996; Herrero et al. 2002; Hirai and Jones 1989; Jones 1985; Morel et al. 1997; Sadikot and Rymar 2009; Walker 1982). In Nissl-stained tissue sections through the human thalamus, the two subnuclei of the human CM-PF exhibit marked differences in their nerve cell densities and staining features. The CM displays a lower cell density than the PF and is richer in fibers with strands and cellular islands irregularly arranged within its boundaries (Fig. 4.4) (Hassler 1982a; Heinsen and Heinsen 1991; Heinsen et al. 1996; Herrero et al. 2002; Hirai and Jones 1989; Jones 1985; Morel et al. 1997; Sadikot and Rymar 2009; Walker 1982).
4.3 Functional Neuroanatomy of the Mediodorsal Nucleus (MD) of the Human Thalamus
The thalamic mediodorsal nucleus (MD) (Fig. 4.1) together with the interconnected prefrontal cortex is known to increase in size and rapidly expands in the ascending phylogeny and, along with the pulvinar, constitutes the phylogenetically most progressed nucleus of the human thalamus (Armstrong 1990; Barbas et al. 1991; Rapoport 1990; Walker 1982). The MD is classified as an associative thalamic nucleus, represents a large aggregation of different subnuclei or regions, occupies wide regions in the central part of the human thalamus, and, after the pulvinar, is the largest nuclear complex in the human thalamus. Most intracerebral connections of the MD are established with the prefrontal cortex (Figs. 4.1 and 4.3). Owing to its reciprocal interconnections with the prefrontal cortex via efferent (i.e., thalamocortical projections from the MD to the inner granular layer IV of the prefrontal cortex) and afferent pathways (i.e., corticothalamic projections from the inner pyramidal layer V and multiform layer VI of the prefrontal cortex to the MD), the MD is considered as a major subcortical link to the prefrontal cortex (Fig. 4.3) (Barbas et al. 1991; Carmichael and Price 1996; Fuster 1989; Giguere and Goldman-Rakic 1988; Haber and Calzavara 2009; Hassler 1982a; Heinsen et al. 1999; Herrero et al. 2002; Jones 1985; Markowitsch 1982; McFarland and Haber 2002; Mesulam 1998; Montoya et al. 2006; Morecraft et al. 1992; Walker 1982; Zilles 1990). Interruptions of these interconnectivities with the prefrontal cortex within the context of neurosurgical prefrontal leucotomy or lobotomy performed to relieve the symptoms of neuropsychiatric disorders are well known to be associated with strong degenerative signs in the human MD (Freeman and Watts 1947; Giguere and Goldman-Rakic 1988; Hassler 1982b; Heinsen et al. 1999; Herrero et al. 2002; Meyer et al. 1947). From a functional point of view, the human MD has been implicated in olfactory processing and cognitive, learning, and memory processes, as well as executive brain functions (e.g., goal formation, planning, goal-directed action, self-monitoring, attention, coordination of complex cognition and motor control for effective performance, dynamical focus change between points of fixation) and oculomotor functions (Barbas et al. 1991; Carmichael and Price 1996; Fuster 1989; Giguere and Goldman-Rakic 1988; Harting et al. 1980; Hassler 1982a; Heinsen et al. 1999; Herrero et al. 2002; Huerta et al. 1986; Jones 1985; Künzle and Akert 1977; Markowitsch 1982; McFarland and Haber 2002; Sommer and Wurtz 2004; Stanton et al. 1988).
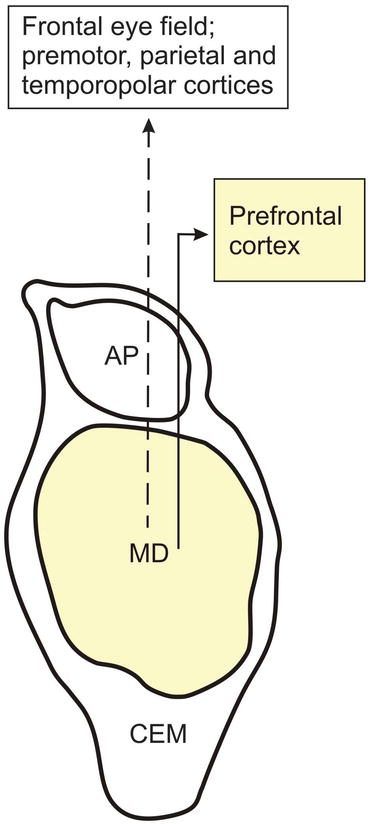

Fig. 4.3
The major projections of the human mediodorsal nucleus (MD) to the cerebral cortex. Diagram of a frontal section through the human mediodorsal thalamic nucleus (MD) showing its main efferent outputs to the cerebral cortex. The brain structures that are known to undergo neurodegeneration during Huntington’s disease (HD) are colored in light yellow. Abbreviations: AP anteroprincipal nucleus of the thalamus, CEM central medial nucleus of the thalamus, MD mediodorsal nucleus of the thalamus
The human MD is surrounded by a split of the internal medullary lamina and a ring of nuclei located within this lamina (Fig. 4.1). The MD is rostrally bordered by the intralaminar central medial nucleus and dorsally by the limbic anteroprincipal and laterodorsal thalamic nuclei, as well as by the small intralaminar cucullar nucleus. The paracentral intralaminar nucleus lies laterally and the midline paraventricular nucleus medially to the MD, while the CM-PF abuts on the MD ventrally (Fig. 4.1). Caudally, the MD borders on the islands of the intralaminar central lateral nucleus and is replaced by the medial pulvinar and the limitans-suprageniculate complex (Figs. 4.1 and 4.5) (Hassler 1982a; Heinsen et al. 1996; Herrero et al. 2002; Hirai and Jones 1989; Jones 1985; Morel et al. 1997; Walker 1982).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







