Patient Factors
Clinician/Practitioner Factors
Systems Factors
• Older subjects
• Patients experiencing comorbid dementia
• Fluctuating course of presentation
• Presence of hypoactive features
• Lack of knowledge and training
• Lack of confidence
• Lack of suspicion
• Lack of time of the clinical staff
• Expectation that altered mental status or delirium are a “normal occurrence” in certain medical settings, such as the ICU
• Lack of consensus over the optimal assessment of delirium
• Location of care [worse in surgical rather than medical settings]
• Busy clinical settings [especially low nurse to patient ration]
• Inadequate application of sedation holidays in sedated-ventilated patients
• The rapid transfer of patients from one unit to another which may decrease the proper documentation and diagnosis
There are a number of clinically available instruments have been developed to screen for the presence of delirium (screening tools, e.g., Confusion Assessment Method [CAM], Confusion Assessment Method for the ICU [CAM-ICU] both based on DSM-III-R criteria), while others allow for a measure of the progression of delirium (severity scales, e.g., Delirium Rating Scale-revised-1998 [DRS-R-98]; Memorial Delirium Assessment Scale [MDAS]; Intensive Care Delirium Screening Checklist [ICDSC], all based on DSM-IV criteria). A significant advantage of diagnostic tools that measure delirium severity is that they provide clinicians a tool to measure the severity of the episode and determine whether the condition seems to be worsening or improving. Severity scales may also provide the ability to diagnose subsyndromal delirium (SSD) (i.e., patients presenting with mental status changes that do not rise to full DSM or ICD diagnostic criteria). Studies suggest that patients suffering from SSD in the general medical wards experienced longer acute care hospital and ICU stay, increased post-discharge mortality, more symptoms of delirium, lower cognitive and functional level at follow-up than patients with no SSD, and greater rate of nursing home placement or death at 6 months post-discharge; even after adjusting for illness severity, and baseline cognitive status and severity of baseline functional status (Marcantonio et al. 2002; Cole et al. 2003, Ouimet et al. 2007a, b).
Unfortunately, no tool is perfect. In fact, a recent study comparing the CAM-ICU and the ICDSC demonstrate an agreement in diagnosing delirium diagnosis between the two methods in only 42 of 162 patients (27.8 %) (Tomasi et al. 2012). Others have demonstrated that the agreement rates between CAM-ICU and ICDSC may vary between different groups of ICU patients (e.g., elective vs. emergency surgery) and seems to be affected by disease severity (Fagundes et al. 2012).
All currently available scales have been derived from and have been validated against expert psychiatric assessments using earlier versions of DSM or ICD diagnostic criteria. It is unclear as of yet, what the new DSM-5 diagnostic criteria will mean to the diagnostic accuracy of existing tools.
As of yet, there are no objective diagnostic tests for delirium. Some have advocated the use of the electroencephalogram (EEG) with its characteristic slowing of peak and average frequencies, and decreased alpha activity, but the clinical usefulness of EEG may be limited by its low specificity (given there are a number of conditions and medications that may affect the EEG) and the impracticality of conducting the test (particularly in the case of agitated and combative patients). Still, the EEG can be useful in differentiating delirium from other psychiatric and neurological conditions such as catatonic states, seizure activity (e.g., non-convulsive status), medication side effects (e.g., posterior reversible encephalopathy syndrome due to the use of calcineurin inhibitors) or the manifestations of the behavioral and psychological symptoms associated with dementia (BPSD). Others have advocated the use of a 24-hr accelerometer-based activity monitor. The continuous wavelet transform (CWT) provided by the instrument can then be used to characterize the phenotypic presentation of delirium as hyperactive, hypoactive, or mixed (Godfrey et al. 2009; Meagher 2009; Godfrey et al. 2010).
12.4 Neuropathogenesis of Delirium
By now it is rather clear that delirium is a neurobehavioral syndrome caused by the transient disruption of normal neuronal activity secondary to systemic disturbances (Engel and Romano 1959; Lipowski 1992; Brown 2000). A number of scientific theories have been proposed in an attempt to explain the processes leading to the development of delirium (Maldonado 2008a, b). Most of these theories are complementary, rather than competing (see Fig. 12.1). It is likely that none of these theories by themselves explain the phenomena of delirium, but rather that two or more of these, if not all, act together to lead to the biochemical derangement we know as delirium (see Table 12.2). A recent detailed review of the most salient theories has been published elsewhere (Maldonado 2013).
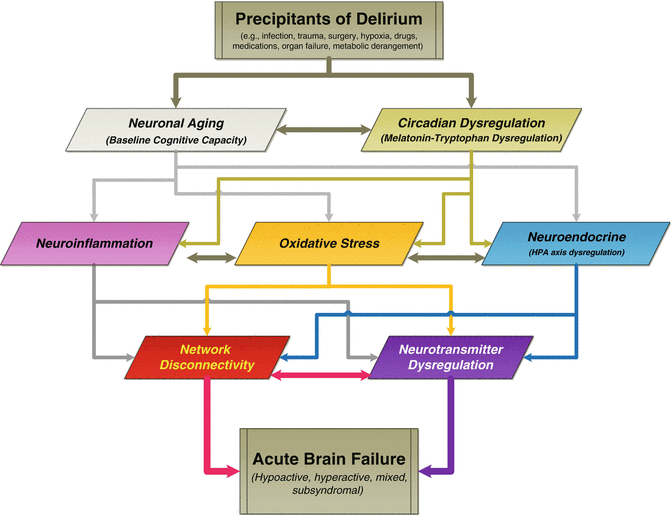

Fig. 12.1
Theories on the Development of Delirium. Schematics of the interrelationship of current theories on the pathophysiology of delirium and how they may relate to each other. Each proposed theory has focused on a specific mechanism or pathologic process (e.g., dopamine excess or acetylcholine deficiency theories), observational and experiential evidence (e.g., sleep deprivation, aging), or empirical data (e.g., specific pharmacological agents’ association with postoperative delirium; intraoperative hypoxia). Most of these theories are complementary, rather than competing, with many areas of intersection and reciprocal influence. The literature suggests that many factors or mechanisms included in these theories lead to a final common outcome associated with an alteration in neurotransmitter synthesis, function, and/or availability, coupled with an acute breakdown in brain network connectivity, leading to the complex behavioral and cognitive changes observed in delirium. In the end, it is unlikely that any one of these theories is fully capable of explaining the etiology or phenomenological manifestations of delirium, but rather that their interaction lead to the biochemical derangement and, ultimately to the complex cognitive and behavioral changes characteristic of delirium. Adapted from (Maldonado 2013)
Table 12.2
Theorized neurochemical mechanisms associated with conditions leading to delirium
Delirium Source | ACH | DA | GLU | GABA | 5HT | NE | Trp | Phe | His | Cytok | HPA axis | NMDA activity | Changes in RBF | EEG | Mel | Inflam | Cort |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Anoxia/hypoxia | ⬇ | ⬆ | ⬆ | ⬆ | ⬇ | ⬇ | ⇔ | ⬆ | ╬⬇ | ╬⬆ | ╬ | ⬆ | ╬ | ⬇ | ⬇ | ⬆ | ⬆ |
Aging | ⬇ | ⬇ | ⬇ | ⬇ | ⬇ | ⬇ | ⬇ | ⬇ | ⬇ | ╬⬆ | ╬ | ⬇ | ╬ | ⬇ | ⬇ | ⬆ | ⬆ |
TBI | ⬆ | ⬆ | ⬆ | ⬆ | ⬆ | ⬆ | ⬆ | ⬆ | ⬇ | ⬆╬ | ⬆ | ⬆ | ⬆ | ⬇ | ⬇ | ⬆ ╬ | ⬆ |
CVA | ⬇ | ⬆ | ⬆ | ⬆ | ⬆ | ⬆ | ⬆ | ⬆ | ⬇ | ⬆╬ | ⬆ | ⬆ | ╬ | ⬇ | ⬇ | ⬆ ╬ | ⬆ |
Hepatic Failure (encephalopathy) | ⇔ | ⬇ | ⬆ | ⬆ | ⬆ | ⬇ | ⬆ | ⬆ | ⬆ | ⬆╬ | ╬ | ⬆ | ╬ | ⬇ | ⬇ | ⬆ | ⬆ |
Sleep deprivation | ⬇ | ⬇ | ╬ | ⬆ | ⬆ | ⬆ | ⬇ | ⬆ | ⬆ | ⬆ | ╬ | ⬆ | ⬆ | ⬇ | ⬇ ╬ | ⬆ ╬ | ⬆ |
Trauma, Sx, and Post-op | ⬇ | ⬆ | ⬆ | ⬆ | ⬇ | ⬆ | ⬇ | ⬆ | ⬆ | ⬆ | ⬆ | ⬆ | ╬ | ⬇ | ⬇ | ⬆ | ⬆ |
ETOH and CNS-Dep Withdrawal | ⬆ | ⬆ | ⬆ | ⬇ | ⬆ | ⬆ | ⬇ | ⬆ | ⬆ | ⬆ | ⬆ ╬ | ⬆ | ⬇ | ⬇ | ⬇ | ⬆ | ⬆ |
Infection/Sepsis | ⬇ | ⬇ | ⬆ | ⬆ | ⬇ | ⬇ | ⬇ | ⬇ | ⬇ | ⬆ | ⬆ ╬ | ⬆ ╬ | ╬ | ⬇ | ⬇ | ⬆ | ⬆ |
Dehydration and Electrolyte Imbalance | ⇔ | ⬆ | ⬆ | ⬆ | ⬇ | ⬆ | ? | ? | ⬆ | ⬆ | ╬ | ⬆ | ⬇ | ╬ | ⬇ | ╬⬆ | ⬆ |
Medical Illness | ⬇ | ⬆ | ⬆ | ╬ | ⬇ | ⬆ | ⬇ | ⬆ | ⬆ | ⬆ | ⬇ | ⬆ | ╬ | ╬ | ⬇ | ╬ | ⬆ |
Regardless of which of these theories is correct (e.g., due to changes in oxidative metabolism, aging, endocrine disturbances, neuroinflammation, changes in sleep–wake pattern), the literature suggests that changes in neurotransmitter concentration or receptor sensitivity are likely to create the substrate conducive to the brain dysfunction characteristic of delirium. In general, the most commonly described neurotransmitter changes associated with delirium include: excess release of norepinephrine (↑ NE), dopamine (↑ DA), and/or glutamate (↑ GLU) and increased Ca + channel activity (↑ Ca + Ch); reduced availability of acetylcholine (↓ Ach) and/or melatonin (↓ MEL); and either a decreased and increased activity in serotonin (↑↓ 5HT); histamine (↑↓ H1&2), and/or gamma-amino butyric acid (↑↓ GABA) likely depending on the etiology or motoric presentation (see Table 12.2). Similarly, it appears that an acute breakdown in brain network connectivity, and the level of inhibitory tone caused by the breakdown, may produce the various motoric phenotypes associated with delirium (e.g., hyperactive, hypoactive, mixed) (Ross 1991; Sanders 2011; Maldonado 2013).
12.5 Etiology of Delirium
Many factors may potentially contribute to the development of delirium. The author uses the mnemonic “End Acute Brain Failure,” to help recall 20 of the most common contributing factors (see Table 12.3). Several factors are discussed here; readers are referred to the author’s comprehensive review of delirium risk factors for further details (Maldonado 2008a, b; Maldonado 2014) Risk factors can be grouped as non-modifiable and potentially modifiable (Table 12.4).
Table 12.3
Delirium: predisposing and precipitating risk factors—“end acute brain failure”
Risk Factor | Examples |
Electrolyte imbalance and dehydration | Electrolyte disturbances (e.g., hyperammonemia, hypercalcemia, hypokalemia/hyperkalemia, hypomagnesemia, hyponatremia/hypernatremia) |
Neurological disorder and injury | All neurological disorders, e.g., CNS malignancies, abscesses, cerebrovascular accident (CVA), vasculitis, multiple sclerosis (MS), epilepsy, Parkinson’s disease, normal pressure hydrocephalus (NPH), traumatic brain injury (TBI), diffuse axonal injury (DAI), limbic encephalitis (both non-paraneoplastic and paraneoplastic syndrome). Of the various forms of sensory impairment, only visual impairment has been shown to contribute to delirium. Visual impairment can increase the risk of delirium 3.5-fold. |
Deficiencies (nutritional) | Nutritional deficiencies (e.g., malnutrition, low serum protein/albumin, low caloric intake, “failure to thrive”), malabsorption disorders (e.g., celiac disease), and hypovitaminosis; specifically deficiencies in cobalamine (B12), folate (B9), niacin (B3; leading to pellagra), thiamine (B1; leading to beriberi and Wernicke’s disorder). |
Age | Age [>65] and Gender [m > f] |
Cognition | Baseline cognitive functioning, including dementia and other cognitive disorders; h/o delirium have all been shown to increase the likelihood of delirium. |
U-Tox (intoxication and withdrawal) | Substances of abuse—Acute illicit substance intoxication (e.g., cocaine, PCP, LSD, hallucinogens), as well as poisons, pesticides, solvents, and heavy metals (i.e., lead, manganese, mercury)—and Substances Withdrawal. |
Trauma Toxins | Physical trauma and injury; heat stroke, hyperthermia, hypothermia, severe burns, including trauma of surgical procedures. Various, including biotoxins [animal poison]; heavy metals (lead, manganese, mercury); insecticides; poisons; carbon dioxide; toxic effect of pharmacological agents [i.e., serotonin syndrome, neuroleptic malignant syndrome, anticholinergic states); Blood levels [toxic levels of various therapeutic substances (e.g., lithium, VPA, carbamazepine, immunosuppressant agents). |
Endocrine disturbance | Endocrinopathies such as hyper/hypo-adrenal corticoid; hyperglycemia/hypoglycemia; hyperthyroidism/hypothyroidism. |
Behavioral-Psychiatric | Certain psychiatric diagnoses, including undue emotional distress; a history of alcohol and other substance abuse, as well as depression, schizophrenia and bipolar disorder have been associated with a higher incidence of delirium |
Rx and other toxins | Several pharmacological agents have been identified, especially those with high anticholinergic activity, including prescribed agents (especially narcotics and GABA-ergic agents)] and various OTC agents [especially anticholinergic substances; polypharmacy] |
Anemia, anoxia, hypoxia, and Low perfusion states | Any state that may contribute to decreased oxygenation (e.g., pulmonary or cardiac failure, hypotension, anemia, hypoperfusion, intraoperative complications, hypoxia, anoxia, carbon monoxide poisoning, shock). |
Infectious | Pneumonia, urinary tract infections, sepsis, encephalitis, meningitis, HIV/AIDS. |
Noxious stimuli (Pain) | Data suggests that both pain and medications used for the treatment of pain have been associated with the development of delirium. Studies have demonstrated that the presence of postoperative pain is an independent predictor of delirium after surgery. On the other hand, the use of opioid agents has been implicated in the development of delirium. |
Failure (organ) | Cardiac, hepatic, pulmonary, and renal failure. |
Apache score (severity of illness) | Evidence shows that the probability of transitioning to delirium increases dramatically for each additional point in the Acute Physiology and Chronic Health Evaluation (APACHE II) severity of illness score. |
Intracranial processes | Stroke (especially non-dominant hemispheric); Intracranial bleed; Meningitis; Encephalitis; Neoplasms |
Light, sleep, and Circadian Rhythm | Sleep deprivation and insomnia, sleep disorders (e.g., obstructive sleep apnea) and disturbances/reversal in sleep–wake cycle. |
Uremia and other Metabolic Disorders | Acidosis, alkalosis, hyperammonemia, hypersensitivity reactions; glucose, acid–base disturbances |
Restraints and any factors causing immobility | The use of restraints, including endotracheal tubes (ventilator),soft and leather restraints, intravenous lines, bladder catheters, and intermittent pneumatic leg compression devices, casts, and traction devices all have been associated with an increased incidence of delirium |
Emergence delirium | Emergence from medication induced sedation, coma or paralysis; which may be associated with CNS depressant withdrawal, opioid withdrawal, REM-rebound, sleep deprivation. |
Table 12.4
Delirium risk factors
Modifiable factors | Non-modifiable factors |
|---|---|
• Various pharmacological agents, especially GABA-ergic and opioid agents, and medications with anticholinergic effects • Prolonged and/or uninterrupted sedation • immobility • Acute substance intoxication • Substance withdrawal states • Use of physical restraints • Water and electrolyte imbalances • Nutritional deficiencies • Metabolic disturbances and endocrinopathies (primarily deficiency or excess of cortisol) • Poor oxygenation states (e.g., hypo-perfusion, hypoxemia, anemia) • Disruption of the sleep–wake cycle • Uncontrolled pain • Emergence delirium | • Older age • Baseline cognitive impairment • Severity of underlying medical illness • Preexisting mental disorders |
Recognition of the non-modifiable factors can help identify patients at high risk for delirium; attention would then turn to those patients’ potentially modifiable factors. Four of the most important non-modifiable factors are older age, baseline cognitive impairment, severity of underlying medical illness, and preexisting mental disorders. Old age is likely a contributor due to increased number of medical comorbidities; overall frailty, decreased volume of ACh producing cells; decreased cerebral oxidative metabolism; cognitive deficits and increased risk of dementia, and age-related cerebral changes in stress-regulating neurotransmitter, intracellular signal transduction systems, and chronic neurodegeneration with an increased production of inflammatory mediators, including cytokines and acute phase proteins. Baseline cognitive deficits even subtle ones, have been associated with an increased the risk of developing delirium. The presence of dementia, more than double the risk for postoperative delirium. Studies have also shown that the severity of the patient’s underlying medical problems has a significant influence in the development and progression of delirium. Finally, the presence of preexisting mental disorders has been associated with an increased rate of delirium (especially bipolar disorder, major depression alcohol use, and anxiety states).
Among the potentially modifiable risk factors, which many be amenable to early intervention and/or prevention include: the use of various pharmacological agents, especially GABA-ergic and opioid agents, and medications with anticholinergic effects; prolonged and/or uninterrupted sedation; immobility; substance intoxication and withdrawal states; the use of physical restraints; water and electrolyte imbalances; nutritional deficiencies; metabolic disturbances and endocrinopathies (primarily deficiency or excess of cortisol); poor oxygenation states (e.g., hypo-perfusion, hypoxemia, anemia); environmental factors impeding adequate rest or causing disruption of the sleep–wake cycle; both the experience of pain and some of the pharmacological agents used for the treatment of pain have been associated with the development of delirium; and the development of emergence delirium (i.e., an altered mental status occurring as a patient “emerges” from deep sedation or medication induced coma).
12.6 Epidemiology
Delirium’s prevalence often surpasses all other psychiatric syndromes in the general medical setting (Maldonado 2008a, b; NICE 2010a, b). Among the “general” population aged 85 + years, the prevalence of delirium is about 10 %, rising up to 22 % in populations with higher percentages of demented elder; and as high as 70 % for those in long-term care (de Lange et al. 2013); making delirium one of the six leading causes of preventable conditions in hospitalized elderly patients (Rothschild and Leape 2000).
The frequency of delirium varies from 15 % to 60 % in hospitalized general medical and surgical patients (Smith and Dimsdale 1989; Francis et al. 1990; Levkoff et al. 1992; Schor et al. 1992; Williams-Russo et al. 1992; Pompei et al. 1994; Parikh and Chung 1995; van der Mast and Roest 1996; Elie et al. 1998; Bucht et al. 1999; Fann 2000; Aldemir et al. 2001; Lepouse et al. 2006; Siddiqi et al. 2006; Hala 2007; Lundstrom et al. 2007; Maldonado 2008a, b; Katznelson et al. 2009; Maldonado et al. 2009; NICE 2010a, b; Tognoni et al. 2011; Ryan et al. 2013). The reported rate of postoperative delirium has been reported to range from 10 % to 74 % (Dyer et al. 1995; Vaurio et al. 2006; Bruce et al. 2007; Maldonado et al. 2009; NICE 2010a, b; Wiesel et al. 2011). Studies have demonstrated that up to 87 % of critically ill patients develop delirium during their ICU stay (Ely et al. 2001a, b). Delirium is common among cancer patients (Adams 1988; Weinrich and Sarna 1994; Morita et al. 2001; Breitbart et al. 2002; Centeno et al. 2004; Gaudreau et al. 2005).
Terminally ill cancer patients, patients with moderate to severe traumatic brain injury, frail elders, and the critically ill are clinical populations generally recognized to have a high incidence of delirium over their course of illness, due to factors such as polypharmacy, comorbid medical conditions, and metabolic dysfunction. Among patients with advanced cancer, delirium was diagnosed in 42 % of patients on admission, while it developed in 45 % of hospitalized cancer patients (not delirious at the time of admission) (Lawlor et al. 2000). Terminal delirium occurred in 88 % of patients dying of cancer (Lawlor et al. 2000).
The large variation of incidence and prevalence data reported reflects differences in patient populations studied and diagnostic criteria used.
12.7 Management of Delirium
The management of delirium has four main components: (1) recognition of patients at risk; (2) implementation of prevention techniques (with pharmacological and non-pharmacological approaches), especially in those populations identified to be at high risk; (3) enhanced surveillance and screening; and (4) treatment of all forms of delirium (with pharmacological and non-pharmacological approaches). In the discussion that follows we combine prevention and treatment methods as some of the pharmacological and non-pharmacological approaches have been shown to be of benefits in both, delirium prevention and amelioration of symptoms and shortening the duration of delirium once it has started (Table 12.5).
Table 12.5
Delirium management algorithm: prevention and management
I.Recognition of patients at risk |
A.A particular patient’s odds of developing delirium are associated with the interaction among the following conditions: |
i.Knowledge of a patient’s characteristic |
ii.Predisposing and precipitating medical risk factors (Table 12.3) |
iii.Modifiable and non-modifiable risk factors for that particular patient or patient population (table 12.4) |
iv.Specific medical conditions and surgical procedures the patient is exposed to |
B.Obtaining the patient’s baseline level of cognitive functioning using information from accessory sources (e.g., IQCODE) |
II.Implementation of prevention techniques |
A.A key focus should be placed on prevention strategies, particularly in “at risk” populations |
i.Avoid all pharmacological agents with high deliriogenic potential or anticholinergic load, if possible |
ii.Promote a non-pharmacological sleep protocol |
iii.Early mobilization (see below for details) |
B.For patients in the ICU, especially those on ventilation or IV sedation, consider: |
i.Sedate to a prescribed or target sedation level (e.g., RASS) |
ii.Consider using the sedative agent with lowest deliriogenic potential: |
• Dexmedetomidine use is associated with the lowest incidence of delirium |
• Propofol use is a good second choice; followed by midazolam |
iii.Reassess pain levels daily and titrate opioid agents to the lowest effective required to maintain adequate analgesia |
• Hydromorphone is preferred as baseline agent of choice for pain management |
• Limit the use of fentanyl for rapid initiation of analgesia and as rescue agent |
• Avoid the use of opioid agents for sedation or management of agitation or delirium |
iv.Provide daily sedation holidays, this includes: |
a.Interrupt sedative infusions daily until the patient is awake |
b.Restart sedation, if needed, at the lowest effective dose |
c.Reassess target sedation level (e.g., RASS) |
III.Enhanced surveillance, screening, and early detection |
A.Most important aspects surveillance: |
i.Knowledge about the condition and presenting symptoms |
ii.A high level of suspicion |
B.Be vigilant for the development of delirium in high risk groups: |
i.Use a standardized surveillance tools (e.g., DRS-R-98; MDAS; CAM) |
ii.Use of psychiatric consultants (i.e., DSM-5/ICD-10 criteria) |
iii.Be particularly aware of the presence of hypoactive delirium and its different manifestation |
C.Use psychiatric consultants to help with assessment and design of the treatment plan, if available |
D.Training of medical personnel at all levels |
IV.Treatment of all forms of delirium |
A.Identify and treat underlying medical causes |
B.Treatment or correction of underlying medical problems and potential reversible factors |
C.Conduct an inventory of all pharmacological agents administered to the patient |
i.Any medication or agent known to cause delirium or to have high anticholinergic potential should be discontinued, if possible, or a suitable alternative instituted |
D.Implement early mobilization techniques, to include ALL of the following components: |
i.Daily awakening protocols (sedation holiday)—as described above |
ii.Remove IV lines, bladder catheters physical restraints and any other immobilizing apparatuses as early as possible |
iii.Aggressive PT and OT as soon as it is medically safe to do |
a.In bedridden patients this may be limited to daily passive range of motion |
b.Once medically stable, get the patient up and moving as early as possible |
iv.Provide patients with any required sensory aids (i.e., eyeglasses, hearing aids) |
v.Promote as normal a circadian light rhythm as possible |
a.Better if this can be achieved by environmental manipulations, such as light control (i.e., lights on and curtains drawn during the day; off at night) and noise control (i.e., provide ear plugs, turn off TVs, minimize night staff chatter) |
b.Provide as much natural light as possible during the daytime |
vi.Provide adequate intellectual and environmental stimulation as early as possible |
E.Avoid using GABAergic agents to control agitation, if possible |
• Exception: cases of CNS-depressant withdrawal (i.e., alcohol, benzodiazepines, barbiturates); or when more appropriate agents have failed and sedations are needed to prevent patient’s harm |
F.Adequately assess and treat pain |
i.Yet avoid the use of opioid agents for behavioral control of agitation |
ii.Rotate opioid agents from morphine to hydromorphone or fentanyl |
G.For the treatment of delirium (all types) consider using: |
i.Acetylcholinesterase inhibitor (e.g., rivastigmine)—for patients with a history of recurrent delirium or delirium superimposed on known cognitive deficits. Physostigmine, for known causes of anticholinergic delirium |
ii.Melatonin (e.g., 3 mg q HS) or melatonin agonists (e.g., ramelteon 8 mg q HS) to promote a more natural sleep. If that is ineffective, consider trazodone (e.g., 25-100 mg q HS) or mirtazapine (e.g., 3.75 – 7.5 mg q HS) |
iii.Serotonin antagonist (e.g., ondansetron) |
H.In case of hyperactive delirium consider the use of the following agents: |
i.Dopamine antagonist agents (to address DA excess) (e.g., haloperidol, risperidone, quetiapine, aripiprazole) |
a.Moderate dose haloperidol (e.g., < 20 mg/24 h in divided doses), is still considered the treatment of choice, if the patient’s cardiac condition allows it |
b.Before using haloperidol: |
• Obtain 12-lead ECG; measure QTc and |
• Check electrolytes; correct K + and Mg+, if needed |
• Carefully review the patient’s medication list and identify any other agents with the ability to prolong QTc |
• If possible avoid other medications known to increase QTc and/or inhibitors of CPY3A4 |
c.When the use of haloperidol is contraindicated or not desirable, atypical antipsychotics should be considered: |
• Better evidence for: risperidone, quetiapine |
• Limited data for: olanzapine, aripripazole, perospirone |
• Avoid: clozapine, ziprasidone |
d.Discontinue dopamine antagonist agents use if QTc increases to >25 % of baseline or >500 msec |
ii.Alpha-2 agonist agents (to address the NE excess) (e.g., dexmedetomidine, clonidine, guanfacine) |
a.Consider changing primary sedative agents from GABA-ergic agents (e.g., propofol or midazolam) to an alpha-2 agent (e.g., dexmedetomidine), starting at 0.4mcg/kg/h, then titrate dose every 20 min to targeted RASS goal |
iii.Anticonvulsant and other agents with glutamate antagonism or Ca + Ch modulation (e.g., VPA, gabapentin, amantadine, memantine) |
F.Consider the use of NMDA-receptor blocking agents, to minimize glutamate-induced neuronal injury (e.g., amantadine, memantine), particularly in cases of TBI and CVA. |
G.In case of hypoactive delirium: |
i.Evidence suggests that DA antagonists may still have a place given the excess DA theory. |
a.If haloperidol is use, recommended doses are in the very-low range (i.e., 0.25 to 1 mg / 24 h). This is usually given as a single nighttime dose, just before sun down |
b.If an atypical is preferred, consider an agent with low sedation (i.e., risperidone, aripiprazole) |
ii.In cases of extreme psychomotor retardation or catatonic features, in the absence of agitation or psychosis, consider the use of psychostimulant agents (e.g., methylphenidate, dextroamphetamine, modafinil) or conventional dopamine agonists (e.g., bromocriptine, amantadine, memantine) |
The recognition of patients at risk begins by knowledge of the patient’s characteristic, an assessment of the predisposing and precipitating medical risk factors the patient is or may be exposed to (Table 12.3), and the modifiable and non-modifiable risk factors for that particular patient or patient population (Table 12.4). Finally, certain medical conditions and surgical procedures are more likely to be associated with the development of delirium than others. It is likely that a particular patient’s odds of developing delirium are associated with the interaction between these four sets of conditions. These have been discussed in detail elsewhere (Maldonado 2008a, b; Maldonado 2014).
Prevention techniques have been found to be rather effective, especially when targeted to patients at high risk for developing delirium. There are a whole host of pharmacological and non-pharmacological techniques available (See Table 12.5). It is important that providers carefully consider the fear of potential side effects versus the benefits associated with effective delirium prevention.
The early recognition of delirium is of upmost importance given the serious negative consequences of misdiagnosis or delayed treatment, including increased morbidity and mortality. A study among ICU patients demonstrated that whose delirium treatment was delayed were more frequently mechanically ventilated (50.0 % vs. 22.3 %; p = 0.012), had more nosocomial infections (including pneumonia) (p < 0.05), and had a higher mortality rate (p < 0.001) than patients whose treatment was promptly started (Heymann et al. 2010).
The most important aspects of an adequate surveillance and early detection include knowledge about the condition and presenting symptoms (of all motor forms) and a high level of suspicion, especially in populations at risk. There are a number of surveillance tools and techniques (discussed above under Diagnosis of Delirium section) that can be used to efficiently screen subjects, especially those at greatest risk. Adequate training of medical personnel at all levels is paramount. Surveillance should be effectively implemented by all practitioners.
Once diagnosed, adequate management of delirium includes the following steps: (1) management of the behavioral and psychiatric manifestations and symptoms to prevent the patient from self-harm or harming of others (e.g., use of tranquilizing agents to manage agitation); (2) treatment or correction of underlying medical problems and potential reversible factors (e.g., correction of electrolyte imbalances, infection, end organ failure, sleep deprivation, pain, metabolic and endocrinological disturbances, substance or medication intoxication or withdrawal); and (3) correction of the neurochemical derangement (triggered by the underlying cause) which leads to the behavioral manifestations of delirium (see Table 12.5).
We advocate an integrative approach which we like to call the “CNS Pharmacotherapy Algorithm” for the prevention and management of delirium in the ICU (Fig. 12.2) (Maldonado 2008a, b; Maldonado 2009; Maldonado 2011). This approach incorporates input and collaboration among all pertinent medical teams including primary treatment team and its consultants (including the CL service), nursing personnel, and members of the various ancillary teams (e.g., respiratory, physical and occupational therapy) and is directed at early mobilization, restoration of the sleep–wake pattern, optimizing patient comfort (i.e., adequate sedation), minimizing pain (i.e., adequate analgesia), prevention or treatment of delirium. This approach is directed at improving physical and cognitive recovery and return to baseline functional level as soon as possible (Fig. 12.3).
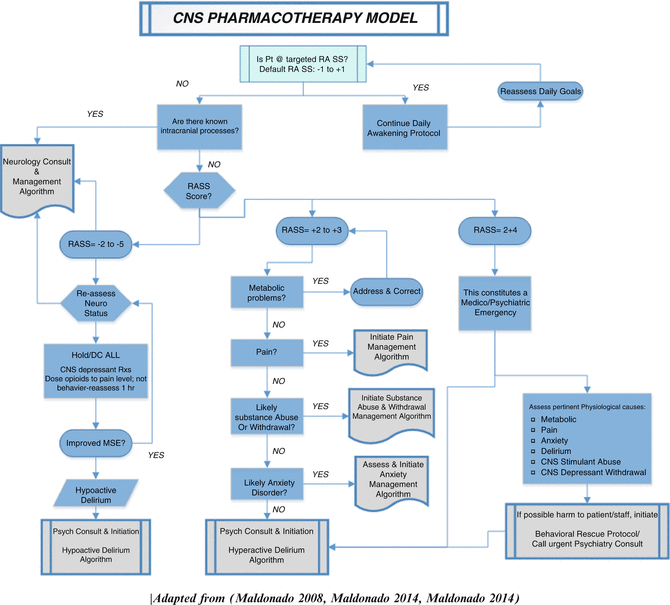
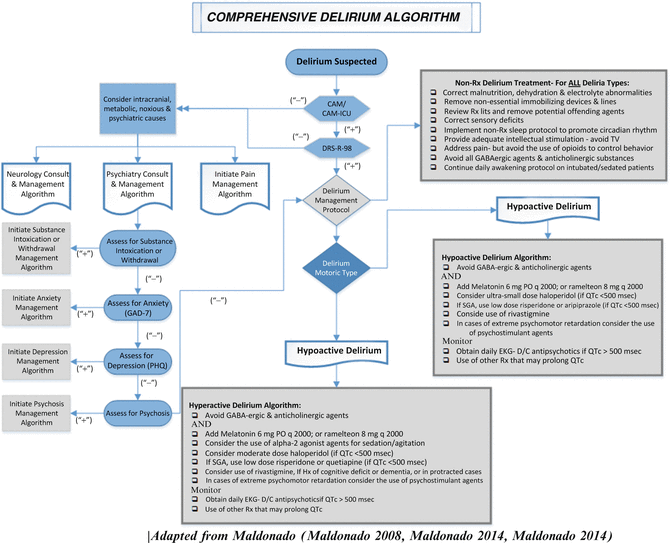

Fig. 12.2
CNS Pharmacotherapy Model

Fig. 12.3
Comprehensive Delirium Algorithm
12.7.1 Non-pharmacological Management Strategies
To date, there are 12 studies published on the use of non-pharmacological prevention strategies in the non-ICU environment, with equal number of studies claiming benefits as those suggesting the intervention has limited or no effect in delirium prevention. The multicomponent protocol consisted of simple techniques applied by the hospital staff, including reorientation, appropriate cognitive stimulation three times a day, the implementation of a non-pharmacologic sleep protocol to help normalize patient’s sleep–wake cycle, early mobilization after surgery or extubation, timely removal of catheters and restraints, correction of sensory deficiencies (i.e., eyeglasses and hearing aids), and early correction of dehydration and electrolyte abnormalities. This has evolved into the Hospital Elder Life Program (HELP) (Inouye and Charpentier 1996, Inouye et al. 1999a, b; Inouye et al. 2000). This program involves the implementation of targeted interventions for known risk factors (i.e., cognitive impairment, sleep deprivation, immobility, dehydration, vision or hearing impairment) for cognitive decline in the elderly by an interdisciplinary team A number of studies have demonstrated the usefulness of the multicomponent approach in preventing delirium (Marcantonio et al. 2001; Tabet et al. 2005; Vidan et al. 2005; Caplan and Harper 2007; Lundstrom et al. 2007; Colombo et al. 2012).
Yet a number of studies have failed to demonstrate the benefits of the multicomponent protocol in delirium prevention (Schindler et al. 1989; Wanich et al. 1992; Milisen et al. 2001; Benedict et al. 2009; Bjorkelund et al. 2010; Gagnon et al. 2012). In fact, recent studies have found that the intervention had no effect on the overall delirium rate (p = 0.84), and made no significant difference on secondary measures (i.e., mean length of hospital stay (p = 0.74), falls (p = 0.43), or discharge to long-term care facilities (p = 0.20) (Holroyd-Leduc et al. 2010a, b). A meta-analysis of published studies found that although the multicomponent interventions appeared to be effective in reducing the incidence of delirium among postoperative patients, it made no difference in a number of important secondary measures, including discharge location or post-discharge dependency, length of hospital stay (p = 0.12), or mortality rate (p = 0.77) between intervention and control groups (Holroyd-Leduc et al. 2010a, b). Of note, a recent study found that the administration of the multicomponent intervention by non-professional, family members lead to a significant reduction in the occurrence of delirium (p = 0.027) (Martinez et al. 2012), suggesting the negative studies mentioned above are a reflection of the mode of administration, rather the approach itself. Of interest, a recent study demonstrated that a relatively simple intervention consisting of daily reorientation, supplemented with environmental, acoustic and visual stimulation significantly lowered the occurrence of delirium (Colombo et al. 2012).
To date, there is only one non-pharmacological intervention that has proven effective in preventing delirium in the intensive care units (ICU). Researchers have found that a combination of early physical and occupational therapy during periods of daily interruption of sedation was accompanied with a significantly greater return to independent functional status (p = 0.02), shorter duration of delirium (p = 0.02), and more ventilator-free days (p = 0.05) (Schweickert et al. 2009). Others have confirmed that a combination of sedation reduction and early mobilization with physical rehabilitation leads to improved outcomes (i.e., lower total amount benzodiazepine use, higher level of functional mobility, and reduction in delirium rates) (Needham and Korupolu 2010).
Finally, some small recent studies have suggested that the use of bright light therapy (i.e., 3000–5000 lux at a distance of 100 cm between light source and patient’s eyes) may be useful in both, prevention (Yang et al. 2012; Chong et al. 2013) and treatment of delirium (Taguchi et al. 2007; Ono et al. 2011). In fact these studies suggest the use of bright light was also associated with an increased mean sleep time (7.7 from 6.4 h; P < 0.05).
In addition, there are a number of steps the primary team can take to effectively decrease the risk of delirium and/or assist in its recovery (Table 12.5). These include conducting an inventory of all pharmacological agents being administered to the patient and discontinuing or substituting potentially offending agents, if possible, especially those with high anticholinergic load. Early discontinuation of all immobilizing lines and devices (e.g., chest tubes, IV lines, bladder catheters), including physical restraints. Early titration of sedation and early mobilization (as described above) has proven to be a key factor in minimizing delirium and improving the odds of return to independent functioning upon discharge home.
Early correction of sensory deficits should be undertaken (e.g., replacement of eyeglasses and hearing aids) as soon as possible. Encouragement of healthy social interaction with family and friends should be made to decrease environmental isolation. Correct malnutrition, dehydration, and electrolyte abnormalities as quickly and safely as possible. Implement environmental manipulations (e.g., increase the amount of natural light during daytime hours, reduce noise levels and artificial lights at nighttime, decrease nighttime tests and procedures) to normalize the sleep–wake cycle and avoid the need of pharmacological sleep agents. In the case of intubated, sedated ICU patients, sedative and pain regimens should be titrated to manage the patients’ symptoms, while allowing for early extubation and mobilization.
The British National Institute for Health and Clinical Excellence (NICE) provided a set of guidelines for the prevention of delirium in elderly at-risk patients, mostly based on the correction of modifiable of factors and the implementation of the multicomponent intervention package (O’Mahony et al. 2011). The full version of these recommendations can be found at <http://guidance.nice.org.uk/CG103/Guidance/pdf/English>.
12.7.2 Pharmacological Management Strategies
While pharmacological agents may assist in the management of agitated patients and in the correction of the neurotransmitter derangements associated with delirium symptoms (Table 12.2), it is important to note that to date no pharmacological agent has received FDA approval for the prevention or treatment of delirium. Also, a systematic review of prospective delirium trials, including prospective randomized and nonrandomized double-blind, single-blind, and open-label clinical trials of any pharmacological agent for the prevention or treatment of delirium demonstrated that pharmacological strategies (e.g., haloperidol, second-generation antipsychotics, gabapentin, melatonin, single dose of ketamine during anesthetic induction, and dexmedetomidine-based sedation compared with other sedation strategies for mechanically ventilated patients) showed greater success in preventing delirium than in treating it (Friedman et al. 2013).
We base the use of pharmacological agents on the premise that the phenomenon of delirium is caused by an underlying metabolic derangement leading to alterations in the neurotransmitter function and the available evidence-based literature. Thus, this section, we address possible pharmacological interventions, based on the neurotransmitter being targeted.
12.7.2.1 Norepinephrine Excess
α 2 -Adrenergic Receptors Agonists versus Conventional Sedative Agents: Effect on Delirium Prevention: There have been several randomized clinical trials looking at anesthetic practice and delirium prevention. Most ICU patients, particularly those who are mechanically ventilated, receive some form of sedation in order to reduce anxiety, encourage sleep and to increase tolerance to the critical care environment, including multiple lines, pain management, endotracheal tubes, and ventilators. Sedative agents (mostly GABA-ergic) and opioids may contribute to the development of delirium by one of six mechanisms: interfering with physiologic sleep patterns; interfering with central cholinergic function; increasing compensatory upregulation of NMDA and kainite receptors and Ca2+ channels; disrupting the circadian rhythm of melatonin release; disrupting thalamic gating function; and leading to CNS depressant dependence and withdrawal (Maldonado 2008a, b).
The author and his team were the first to report on the use of the novel sedative agent, dexmedetomidine (DEX) as an alternative to the use of benzodiazepines and related agents (e.g., midazolam (MID), propofol (PRO)) during the postoperative state (Maldonado et al. 2003a, b). We studied patients (n = 118) undergoing cardiac surgery (i.e., repair or replacement) with cardiopulmonary bypass (CPB) (Maldonado et al. 2003a, b; Maldonado et al. 2009). After successful weaning from CPB, patients were started on one of three randomly assigned, postoperative sedation regimens: dexmedetomidine (DEX), propofol (PRO), or midazolam (MID). There were no significant preoperative or intraoperative differences between treatment groups (e.g., age, sex, ASA classes, bypass time, clamp time, or lowest temperature achieved), except for the type of postoperative sedation. The study found an incidence of delirium of 3 % for patients on DEX, compared to 50 % for propofol or midazolam (p < 0.01) suggesting a “delirium-sparing effect.” Similarly, the number of delirious days was also significantly lower in the DEX group compared to PROP and MID (1 % vs. 16 % vs. 29 %, respectively; p < 0.001) (Maldonado et al. 2009).
Subsequent DBRPCTs have confirmed the original findings and demonstrated the delirium sparing effects of dexmedetomidine (DEX) as compared to conventional sedation (i.e., midazolam) (Pandharipande et al. 2007; Reade et al. 2009; Riker et al. 2009; Shehabi et al. 2009; Jakob et al. 2012). There is evidence suggesting that other α2-agonists (e.g., clonidine) may have similar deliriolytic effects (Rubino et al. 2010). Despite evidence demonstrating that prophylactic use of dexmedetomidine in reducing the incidence of delirium and plenty of clinical evidence that it is useful in the management of agitation related to hyperactive delirium, there is no study to date confirming its potential in the treatment of delirium.
12.7.2.2 Dopamine Excess
Dopamine Antagonists for Delirium Prevention: Of the seven studies published on the use of various antipsychotic agents for delirium prevention five demonstrated positive results (i.e., the Intensity and duration of postoperative delirium were more severe and lasted longer in the control group) (Kaneko et al. 1999; Prakanrattana and Prapaitrakool 2007; Larsen et al. 2010; Wang et al. 2012; van den Boogaard et al. 2013) Of note, two of the studies showing the most significant effects used relatively small doses of risperidone or olanzapine (a single dose preoperatively; or a dose pre-op and a second dose immediately after surgery, respectively) (Prakanrattana and Prapaitrakool 2007; Larsen et al. 2010).
Of the negative studies, one compared haloperidol (0.5 mg/d preoperatively and until postoperative day #3) to placebo, also in at-risk patients, elderly orthopedic patients (Kalisvaart et al. 2005). Although the incidence of delirium was not significantly lower, the use of haloperidol was associated with lower severity (p < 0.001) and shorter duration (p < 0.001) of delirium and shortened length of hospital stay (p < 0.001).
Two recent meta-analyses of studies using dopamine antagonist agents for delirium prophylaxis found that pooled relative risk of published studies suggested a 50 % reduction in the relative risk of delirium among those receiving antipsychotic medication compared with placebo (p < 0.01). The studies suggest that perioperative use of prophylactic dopamine antagonist agents (both typical and second generation antipsychotics), when compared to placebo, may effectively reduce the overall risk of postoperative delirium, thereby potentially reducing mortality, disease burden, length of hospital stay, and associated healthcare costs (Hirota and Kishi 2013; Teslyar et al. 2013)
Dopamine Antagonists for Delirium Treatment: Intravenous neuroleptic agents have been the treatment of choice for agitated and mixed type delirium, particularly in the ICU (Adams et al. 1986; Fernandez et al. 1988; Sanders et al. 1989; Ziehm 1991; Riker et al. 1994; Inouye et al. 1999a, b). Similarly, a number of national and international organizations (i.e., Britain’s National Institute for Health and Clinical Excellence (NICE 2010a, b); American Psychiatric Association (Association 1999); Society of Critical Care Medicine (SCCM) (Shapiro et al. 1995; Jacobi et al. 2002)) have recognized IV haloperidol as the agent of choice for the management of critically ill delirious patients. Similarly, a “best evidence topic in cardiac surgery” suggested that haloperidol should be considered the first line drug for agitated patients post cardiac surgery (Khasati et al. 2004). Keep in mind that haloperidol has never been approved by the FDA for IV use and that the Federal Drug Administration (FDA) issued a “black-box” warning for the “off-label” clinical practice of using IV-haloperidol (FDA 2007).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



