Fig. 11.1
Examples of stainings visualising meningitis-causing agents. Upper panel: May–Grünwald–Giemsa-stained cytospin for cytological differentiation of CSF cells can already visualise bacteria or fungi, and thorough inspection is mandatory: (a) mostly intracellularly located diplococci, culturally confirmed Neisseria meningitides; (b) extracellularly located diplococcic, culturally confirmed Streptococcus pneumonia; (c) budding yeast surrounded by a capsule, culturally confirmed Cryptococcus neoformans. Lower panel: Pathogen-specific staining for further characterisation: (d) Gram-negative, mostly intracellularly located diplococci, culturally confirmed Neisseria meningitides (same patient as upper panel a); (e) Gram-positive bacilli, culturally confirmed Listeria monocytogenes; (f) Indian ink visualising the capsule of Cryptococcus neoformans in CSF (same patient as upper panel c) (Special thanks to Ingelore Nagel for images and continuous support)
In acute bacterial meningitis, Gram staining of CSF preparations allows rapid diagnosis in 60–90 % of cases. However, the sensitivity varies between the pathogen species between 90 % in pneumococcal meningitis and <50 % in CNS infection with Listeria monocytogenes (Tunkel and Scheld 2005). Likewise, sensitivity depends on the preparation and staining technique. Cytocentrifuge preparations showed greater sensitivity in comparison to smears after conventional centrifugation (Chapin-Robertson et al. 1992). Low bacterial CSF load, of course, results in lower sensitivity than high bacterial load; microscopical detection can be achieved in samples with bacterial cell counts below <103 CFU/ml (colony-forming units per ml) in 25 %, in samples with >105 CFU/ml in 97 % (La Scolea and Dryja 1984). After onset of antibiotic treatment, sensitivity of Gram-stained smear is reduced to 40–60 %, and sensitivity of cultural detection is reduced to less than 50 %.
In children, effective antibiotic treatment results in sterilisation of initially positive CSF cultures after 24–36 h (Bonadio 1992). Due to this reason, it should never be missed to draw blood cultures and in suspected meningococcal meningitis also pharyngeal swabs and samples of petechial skin lesions prior to start of antibiotic treatment.
In suspected tuberculous meningitis, CSF cell sediment should be analysed after Ziehl–Neelsen staining with a low sensitivity of about 30 % (Erdem et al. 2013), but the sensitivity can be increased by repeated lumbar punctures for CSF analysis (Kennedy and Fallon 1979).
Fungal CNS infections occur in Europe mainly in immunocompromised patients with especial importance of the yeasts Cryptococcus and Candida and the mould Aspergillus. Meningitis due to Cryptococcus neoformans is one of the most frequent opportunistic infections in patients with impaired cellular immunity. Sensitivity of microscopic detection of cryptococci after CSF staining with Indian ink or May–Grünwald–Giemsa is with 80–90 % high (Zhang et al. 2014; Saha et al. 2009); cultural methods and antigen detection in CSF and serum are important, too (Perfect 2005; Marchetti et al. 2012).
11.3 Antigen Tests
Detection of antigens of pathogenic agents within the CSF can be a helpful supplement for diagnosing bacterial and fungal CNS infections. These tests are based upon the principle to detect antigens by antibody-coated latex particles that will agglutinate with specimens containing the respective pathogens. The advantage of these tests is the rapid procedure and result. Sensitivity is only high in case of a high pathogen load in the CSF, and by this way, the sensitivity of antigen tests is not superior to microscopy after pathogen-specific CSF staining.
Bacterial antigen tests are available to detect Streptococcus pneumoniae, Neisseria meningitidis (serotypes A, B, C, Y, W135), group B streptococci and Escherichia coli K1. There is a certain benefit of bacterial antigen tests to confirm diagnosis and bacterial species in case of antibiotic pretreatment that lowers the sensitivity of bacterial culture; the method should be used in sense of confirmation and not exclusion. As the diagnostic value of bacterial antigen tests has been questioned repeatedly, there are no clear recommendations (Tunkel et al. 2004) and many laboratories have discontinued the use of the bacterial antigen tests. In any case, additional routine microbiological analyses including microscopy and cultural methods are mandatory.
In contrast, in the diagnosis of cryptococcal meningitis, the use of cryptococcal antigen test is clearly recommended in addition to staining and fungal culture (Portegies et al. 2004; Arendt 2012). The test detects polysaccharide antigens of the encapsulated yeast Cryptococcus neoformans in serum and CSF (Bennett et al. 1964), and its sensitivity is very high (in CSF 95–99 %) in comparison to fungal culture.
11.4 Microbiological Culture
Microbial cultures are the gold standard to determine the pathogenic agent in bacterial or fungal CNS infection. Beyond the determination of the type of organism, it allows the detection and quantification of the strain-specific susceptibility to antibiotic or antifungal drugs.
Most bacteria that cause acute meningitis grow well on solid or fluid culture media like blood and chocolate agar or tryptic soy broth, unless they have been damaged by antibiotic intervention (Gill et al. 2005). Selective culture media are, for example, Sabouraud agar suitable for the cultivation and differentiation of fungi or Löwenstein–Jensen medium, specially used for culture of Mycobacterium.
CSF specimens drawn for suspected acute bacterial meningitis will be cultivated for at least 72 h at 35–37 °C and in CO2-enriched aerobic atmosphere. Other suspected CNS infections, as fungal or tuberculous meningitis, require different and specialised growth media and conditions (selective media, special temperatures, longer cultivation time). Therefore, it is of outstanding importance that the treating physician informs the microbiological laboratory about the suspected diagnosis.
In any case of suspected bacterial meningitis, it should be kept in mind to draw specimens for aerobic and anaerobic blood cultures prior to antibiotic treatment.
11.5 Molecular Assays
Amplification techniques such as those using PCR provide increased sensitivity and specificity with short processing time because of the rapid and extensive amplification of target nucleic acid. They are method of choice in the diagnosis of numerous viral CNS infections and improve the diagnosis of bacterial, tuberculous and parasitic infections and can detect, for example, herpes simplex virus (HSV) DNA by amplification of below ten copies DNA per ml. Due to the high sensitivity of PCR, care must be taken to avoid contamination with other templates and amplicons that may be present in the laboratory environment; thus, strict intra-laboratory quality standards are necessary to ensure accurate results. In addition to the high clinical utility of PCR in identifying aetiologic agents of CNS disease, also quantification of viral load to monitor duration and adequacy of antiviral drug therapy (e.g. HIV) is routinely used.
In most PCR protocols and assays, DNA or RNA is extracted from a sample volume of about 100–200 μl (Debiasi and Tyler 2004). For CSF analysis in suspected tuberculous meningitis, relevant larger sample volumes up to volumes >6 ml CSF are recommended (Thwaites et al. 2004).
In general, endogenous polymerase inhibitors are much less commonly present in CSF than in other body fluids or tissues. Nevertheless, false-negative results also occur in CSF analysis. Factors, which might contribute to low sensitivity, include low viral load, delay in CSF processing or rapid clearance due to robust host neutralising antibody response. False-negative results may also occur in the presence of endogenous polymerase inhibitors; especially haeme products from artificial blood contamination may inhibit the PCR, and this should be kept in mind in case of unexpected negative results as in suspected herpes simplex encephalitis (Debiasi and Tyler 2004).
Important applications of PCR-based CSF analytics are suspected herpes simplex encephalitis caused by HSV-1 or less common HSV-2, CNS infections by varicella zoster virus (VZV), Epstein–Barr virus (EBV), cytomegalovirus (CMV), enteroviruses or the polyomavirus JC virus causing progressive multifocal leukoencephalopathy (PML).
In herpes simplex encephalitis, the sensitivity of PCR-based assays is above 95 % with likewise high specificity of 99 %. HSV DNA can still be detected after onset of antiviral treatment for at least 5–7 days. In this early stage of the disease, serological assays to detect intrathecal antibody synthesis against HSE are not of clinical relevance. However, within the first 72 h of herpes simplex encephalitis, false-negative results occur. In well-founded clinical suspicion of herpes simplex encephalitis, antiviral treatment must not be interrupted because of a negative PCR result. Negative results should be interpreted with particular caution, if the CSF specimen was sanguineous or xanthochrome (Tyler 2004; Steiner et al. 2012).
Also diagnostic of bacterial CNS infections is improved by PCR methods. The availability of results (within 8–24 h) is faster in comparison to conventional culture methods, for which results usually are not available for 48 h. Real-time multiplex PCR methods detect H. influenzae, N. meningitidis, S. pneumoniae and L. monocytogenes with high sensitivity (87–100 %) and specificity (98–100 %) (Chaudhuri et al. 2008). PCR methods are of special interest in specimen with delayed processing time or sampling after onset of antibiotic treatment, in which sensitivity of microscopy and bacterial culture decreases. In cases of uncommon or unsuspected bacterial agents, broad-range bacterial PCR assays can be useful in detecting the bacterial gene coding for the 16S ribosomal ribonucleic acid, followed by DNA sequencing for species identification (Greisen et al. 1994; Srinivasan et al. 2012). In suspected neurotuberculosis, PCR assays are supplemental diagnostic tools, especially due to the rapid availability of results in comparison to the long duration of cultivation of up to 6 weeks. But the reported sensitivities of PCR-based tests for Mycobacterium tuberculosis in CSF samples range from 46 to 66 % and specificities from 97 to 99 %. Thus, results must be interpreted with caution (Steiner et al. 2012).
11.6 Serology
Besides the methods for direct detection of pathogens, serological tests play an important in role in diagnosing and confirming CNS infections. Serological tests to identify pathogen-directed antibodies in serum and CSF are especially important in subacute or chronic infectious diseases, as, for example, neuroborreliosis or the second phase of the biphasic presenting tick-borne encephalitis. In contrast to the acute infections in bacterial meningitis and herpes simplex encephalitis, clinical signs of CNS involvement present weeks after infection.
For example, neuroborreliosis is diagnosed by the combination of typical neurological symptoms, parameter of acute meningeal inflammation within the CSF (pleocytosis, blood–CSF barrier dysfunction) and Borrelia burgdorferi-specific antibodies produced intrathecally. Serological evidence of intrathecal immune response by detection of B. burgdorferi-specific IgG and IgM antibody index (AI) values is gold standard in the diagnosis of neuroborreliosis and has a sensitivity of 80 % (duration of infection <6 weeks) to nearly 100 % (>6 weeks). Due to its low sensitivity of only 10–30 %, B. burgdorferi PCR is not of significant clinical relevance and should be limited on very early stages of disease or in patients with immunosuppression (Mygland et al. 2009).
Tick-borne encephalitis (TBE) is a characteristic biphasic febrile illness, involving the central nervous system with symptoms of meningitis, encephalitis or meningoencephalitis. After an asymptomatic incubation period between 7 and 14 days, an initial phase of fever and non-specific symptoms occurs. After about 1 week of remission, CNS affection occurs in 10–30 % of patients. CSF analysis reveals signs of acute viral infection with pleocytosis, blood–CSF barrier dysfunction and intrathecally produced antibodies against TBE virus by detection of TBEV-specific antibody index values (Holzmann 2003).
11.6.1 Detection of Antigen-Specific Intrathecal Immune Response
Intrathecal synthesis of total IgG, IgA and IgM can be detected by immunonephelometric determination of the CSF-to-serum ratios of IgG, IgA, IgM and albumin and plotting IgG, IgA and IgM ratios versus albumin ratio according to the nomogram of Reiber and Felgenhauer (Reiber and Felgenhauer 1987) (see also Chap. 10).
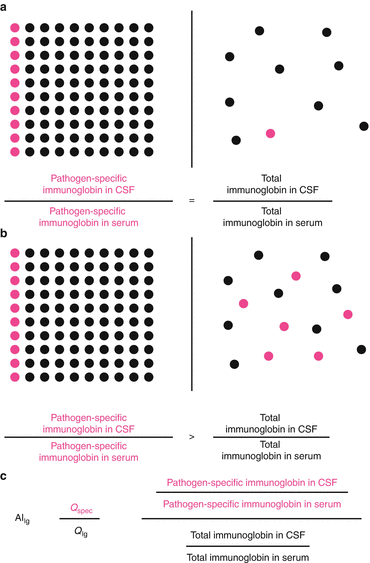
To detect a pathogen-specific intrathecal antibody synthesis, calculation of antigen-specific antibody index (AI) values is commonly used (Reiber and Lange 1991). The determination of specific antibody index values allows detecting a pathological, brain-derived fraction of specific antibodies in CSF, not deriving from serum by flow-depended diffusion via the “blood–CSF barrier”. Species-specific immunoglobulins deriving from serum enter the CSF at equal proportion as total immunoglobulin of the same isotype (Fig. 11.2a). Therefore, the quotient of arbitrary or absolute concentration of pathogen-specific immunoglobulin in CSF divided by concentration of pathogen-specific immunoglobulin in serum (Q spec) has to be the same as the quotient of total immunoglobulin concentration in CSF divided by total immunoglobulin concentration in serum (Q Ig). As the antibody index is the ratio of Q spec divided by Q Ig (Fig. 11.2c), the resulting antibody index is 1. In case of intrathecal immunoglobulin synthesis (Fig. 11.2b), the pathogen-specific antibody concentration ratio (Q spec) increases in relation to the total antibody concentration ratio (Q Ig) resulting in elevated antibody index values above 1.