Development of Cortical Excitability
Peter B. Crino
Introduction
In the human brain, cerebral cortical development spans approximately weeks 7 to 24 of gestation and proceeds from a primordial neural tube structure containing a seemingly unspecified pseudostratified ventricular zone (VZ) epithelium to a mature layered cortex containing virtually all of the necessary cellular elements required for a functional cortex in the behaving animal. The assembly of each cortical layer is a spatiotemporally regulated process in which a series of well-orchestrated molecular events culminate in the formation of a six-layered cytoarchitecture. During this time, many early structural connections will herald functional capabilities such as the formation of synaptic connections, expression of neurotransmitter receptor subunits or ion channels, generation of axon potentials, and, ultimately, electrical activity. Indeed, it is virtually axiomatic that the development of functional electrical excitability in the cortex is necessary for virtually all cortical activity in the mature animal. One consequence of the disruption of these functional attributes is altered cellular excitability and, potentially, epileptogenesis. Thus, understanding the developmental assembly of structural elements that result in normal cortical excitability can highlight possible mechanisms leading to seizures and epilepsy. Indeed, several well-defined epilepsy syndromes result from single gene defects that alter the appropriate temporal expression of a single neurotransmitter receptor subunit or ion channel. This chapter will address the development of cortical excitability as a function of structural alterations such as neuronal cytoarchitecture and pharmacologic changes including neurotransmitter receptor and ion channel expression.
Cortical Development: Structural Changes
Early Cortical Development: Overview
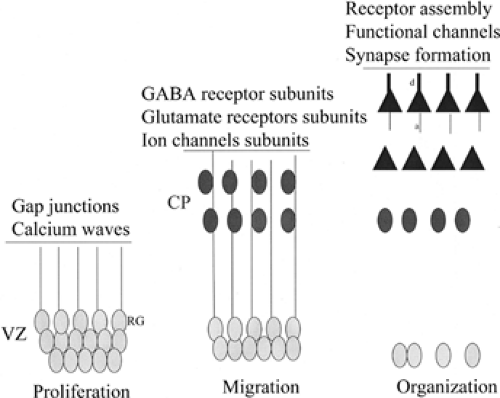
At each cortical developmental epoch, there is progressive assembly of cellular, subcellular, and pharmacologic machinery necessary for mature cortical function.37 Thus, while cells in the VZ and early cortical plate are linked by nonsynaptic electrotonic coupling (e.g., gap junctions), following the establishment of cortical laminae, the formation of axodendritic synaptic connections and transcriptional expression of many neurotransmitter receptor subunits and voltage-gated ion channels permits synaptic transmission (Fig. 1). Concurrent with this process, additional cell types such as astrocytes and oligodendrocytes appear in the cortex and refine synaptic transmission. The ingrowth of afferent fibers from brainstem monoaminergic nuclei (e.g., the locus coeruleus and raphe complexes) as well as cholinergic input from the basal forebrain contribute to modulation of synaptic function. Perhaps most interesting is that the establishment of synaptic machinery does not end with birth but continues into postnatal periods. Thus, the development of normal cortical excitability spans embryonic and postnatal periods. The implications of this time course for epileptogenesis are profound since subtle or overt disruptions in either structural assembly or pharmacologic regulation can in theory lead to epileptogenesis.
Assembly of the Cerebral Cortex
Cortical development occurs in three broad stages: Proliferation, migration, and organization. Proliferation of progenitor cells that will populate the cortex occurs in two primary sources: (a) a single layer of mitotic progenitor cells in the VZ gives rise to most excitatory projection neurons (pyramidal cells19) and (b) progenitor cells in the ganglionic eminence give rise to most local circuit, inhibitory interneurons.26,34 During the proliferative phases, radial glial cells serve as progenitor cells in the VZ of the neural tube and undergo active mitosis, giving rise to daughter progeny.19 Cells destined for a specific cortical layer are born during similar and restricted developmental epochs. Mitotic progenitors express select genes such as intermediate filaments (nestin, vimentin), transcription factors (OTX-1, Emx1, Pax6, BF1/BF2, HES-1), and markers of active cell cycle phases such as proliferating cell nuclear antigen (PCNA) and cyclins (cyclin A, D1). Daughter progeny of progenitor cells either continue to divide or exit the cell cycle (become postmitotic) and differentiate into mature neurons. At this point, cell cycle G0, expression of many “embryonic” genes is down-regulated and a new set of “mature” cellular genes are turned on as cells initiate migration and lamination.
The evolving cortical plate is formed between the marginal zone (the future layer I) above and subplate below.34,35 During the migratory phase, differentiated daughter cells exit the VZ and move radially into the cortical plate along radial glial fibers in an inside-out gradient (i.e., neurons destined for deeper cortical layers [VI] exit the VZ first, followed in succession by waves of neurons destined for more superficial laminae [V to II26]). Progenitor cells deriving from the ganglionic eminences follow nonradial (tangential) paths to the cortex using trophic and other guidance signals.42 Select genes are turned on (e.g., doublecortin) that regulate physical migration of individual cells.
During the organizational phases of cortical development, upon arrival into the appropriate cortical layer, neurons extend dendrites and axons and receive a variety of afferent inputs. At this time, synaptic connectivity is initiated. At the molecular level, new expression of many distinct genes including structural proteins necessary for dendrite and axon outgrowth and synapse formation permits the generation of nascent synapses in the late embryonic and early postnatal cortex. Finally, a substantial proportion of neurons within both the cortical plate and subplate undergo apoptosis.
In the earliest phases of cortical development, there is little spontaneous electrical activity and, in fact, the formation of synapses with subsequent synaptic transmitter release does not occur until later developmental epochs.30,31 During migratory and early organizational phases of development, much of the structural machinery necessary for synaptic connections have not yet formed and other important components of synaptic function (i.e., glia) have not yet arrived in the cortical plate. In the VZ, there is electrotonic coupling between cells mediated largely by gap junctions.28 Calcium waves spread selectively through the VZ, a region rich in radial glial cell bodies, and the majority of participating cells are radial glia.40 Several investigators have demonstrated that calcium waves propagate through the proliferative zone of the embryonic cortex mediated via gap junction proteins (connexins) and intracellular calcium release.40 In the rodent, VZ calcium waves become more robust from embryonic day 12 (E12) to E16 and coincide with increasing levels of neurogenesis. In addition to setting the stage for early electrical activity in cortex, calcium waves may be involved in modulating neurogenesis during embryonic cortical development. Thereafter, calcium waves abate in the postnatal brain and are replaced by synaptically mediated potentials in the nascent cortex.
Cajal-Retzius Cells
One exception to migratory schema of the embryonic cortex are Cajal-Retzius (CR) cells, which are present in the marginal zone before other neurons initiate migration from either the VZ or ganglionic eminences. CR cells represent a transient population of layer I cells present at early stages of cortical development that diminish during postnatal development.24 The secreted protein reelin is released by CR cells and plays a pivotal role in permitting appropriate lamination. Layer I in the developing neocortex consists of CR and non-CR cells. Most non-CR cells are γ-aminobutyric acid (GABA)-ergic, whereas CR cells are glutamate immunoreactive but not GABA immunoreactive.24 CR cells also express parvalbumin and calbindin. CR cells are first observed in the preplate and later in the marginal zone. CR cells respond to various neurotransmitters and contribute to the synchronized network activity in layer I of the neocortex in which the apical dendrites of many deep and superficial layer pyramidal cells will arborize.33 CR cells receive dense GABAergic and non-GABAergic input on cell body and dendrites.20 CR cells express GABAA receptors and NR1/NR2B subunit-containing receptors.33 Corticogenesis may depend on the early electrical activity of CR cells and, in fact, CR cells express functional Ca2+ channels. Recent calcium imaging analysis has shown that the developing layer I neurons exhibit correlated neuronal activity that could serve as the scaffold for the activity-dependent development of intracortical connections.20
Dendritic Development
Dendrites play a crucial role in synaptic signal integration, synaptic plasticity, and network connectivity.32 Dendritic arborization on pyramidal and local circuit neurons occurs progressively beginning in late embryonic stages of development and extending well into the postnatal periods. Extension of dendritic arbors is a pivotal process for subsequent connectivity since synaptic connections between dendrites and incoming axonal afferents drives many of the functional properties of the mature cortex. Many dendrites of pyramidal neurons in deep layers arborize in layer IV, while some extend to more superficial layers. Pyramidal cell dendrites in layer III arborize in layer I. Thalamocortical afferents will make synaptic contact with dendrites in layer IV, while myriad other inputs will meet dendrites in layer I. The establishment of synaptic structure (see below) is in part driven by signals from incoming afferent fibers, and throughout life there is a high degree of structural and functional plasticity in axodendritic synapses. Several factors, both extrinsic and intrinsic to the neuron, are involved in the regulation of dendritic size. These factors include genetic control, electrochemical signals from incoming axons, and release of growth or trophic factors.
Synapse Formation
The generation of functional synapses in the cortex is critical for the development of cortical excitability, and a complete review of this topic is beyond the scope of any single chapter. In rodents, neocortical inhibitory synaptogenesis is a protracted process that begins in late embryonic life and matures through the first postnatal month. In man, synaptogenesis begins early prenatally and peaks a few months after birth. Perhaps the most striking physical feature of the developing cortex is the precision of its synaptic connections since these specifications herald cortical excitability.17 In rodent, nascent synapses first appear in the late embryonic periods, but the numbers dramatically increase over early postnatal periods, coincident with the overwhelming flood of activity generated by activity and experience in the postnatal period. From a molecular perspective, the molecules governing synapse formation can be classified into at least two types: Synaptic recognition molecules that mediate
choices of appropriate synaptic partners and synaptic organizing molecules that regulate maturation and differentiation of the synapse. Thus, synapse formation is driven by internal cellular programs but also in response to activity-dependent cues from incoming axons.
choices of appropriate synaptic partners and synaptic organizing molecules that regulate maturation and differentiation of the synapse. Thus, synapse formation is driven by internal cellular programs but also in response to activity-dependent cues from incoming axons.
As axons and dendrites meet, a complex set of mechanisms set the stage for functional connectivity.32 From a structural perspective, the pre- and postsynaptic elements fuse to form the synaptic cleft. There is rapid expression of cytoskeletal and structural proteins (e.g., cadherins, neuroligins, and GAP-43) to solidify the axodendritic contact. Subsequent expression of synaptic vesicle proteins such as syaptophsyin, synaptotagmin, and synaptobrevin permits formation of presynaptic vesicles for neurotransmitter release, whereas expression of postsynaptic proteins such as dendritic postsynaptic protein 95 (PSD95) or gephyrin are necessary for neurotransmitter receptor subunit assembly into the cell membrane. Ultimately, expression and targeting of neurotransmitter receptor to the synaptic cleft will lead to the establishment of a functional synapse.
Cortical Development: Pharmacologic Changes
Over the course of late embryonic and early postnatal development progenitor cells migrate to form cortical layers and then extend axons and dendrites. Concurrent with these events, there is the appearance of multiple and distinct neurotransmitter receptor subunits and ion channels that mediate functional synaptic connectivity (Fig. 1). The major neurotransmitter systems include the glutamate and GABA systems, and the primary ion channels include calcium, sodium, and potassium channels. Of course, other molecules such as neuropeptides, neurohormones, and unique compounds such as adenosine and nitric oxide play important though not fully defined roles in the development of cortical excitability.
The Glutamate System
Glutamate is the primary excitatory neurotransmitter in the brain and its effects are mediated via two excitatory receptor subtypes: Inotropic and metabotropic receptors (see Chapter 22). The ionotropic glutamate receptor family can be further divided into the N-methyl-D-aspartate (NMDA) receptor (NMDAR) and the non-NMDA receptor (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionate [AMPA] receptor [AMPAR] and kainate receptor) subfamilies. The NMDA, AMPA, and kainate subtypes are ligand-gated, cation-selective channels consisting of multiple protein subunits. The expression of these subunits, like GABA receptors, fluctuates during embryogenesis and early postnatal development. All of these subtypes exhibit enhanced expression from embryogenesis to early postnatal life. In the rodent, AMPA and NMDA receptors exhibit a peak in expression in early postnatal life but then diminish to a relatively constitutive level with further maturity. Glutamate receptors have been implicated in synaptogenesis, learning, and memory and likely are pivotal in the establishment of normal cortical excitability.
The NMDA receptor complex consists of two NR1 subunits (which have eight splice variants) and up to two of four NR2 subunits (NR2A to NR2D). Functional NMDARs are heteromeric complexes, permissive for calcium, and composed of at least one NR1 subunit and one or more NR2A to NR2D subunits, the presence of which modulates channel functional properties. The NR2 subunits confer distinct pharmacologic and kinetic properties on the receptor. The subunit composition of all ionotropic glutamate receptors changes during development and varies in different regions of the mature brain.27 It is likely that particular NMDA receptor subunit combinations underlie different functions of the receptor and regulate synaptic plasticity during brain development.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree