and Róbert Bódizs2
(1)
Institute of Experimental Medicine and Institute of Neuroscience, Budapest, Hungary
(2)
Semmelweis University Institute of Behavioral Science, Budapest, Hungary
Abstract
A main part of fundamental knowledge about sleep regulation roots in the recognition of reciprocally antagonistic sleep-wake-promoting influences originating in brainstem/hypothalamic neuronal networks and in their dynamic interplay. All the presently known concepts and models of sleep regulation are essentially based on the anatomy and function of these subcortical networks. From the beginning of this century, the Harvard School of sleep research and the Lyon sleep research group have introduced important concepts about the working of the flip-flop model of “sleep switch” governing sleep/wake alternations and tried to connect these views with the earlier ideas explaining sleep cyclicity. These results have suggested that the subcortical neuronal assemblies have indeed a governing role in the EEG and behavioral changes during the sleep/wake cycle. However, within the NREM sleep period, robust dynamical changes take place, about which the flip-flop model has nothing to say about. NREM sleep cannot be taken as a “stable” state. Growing evidences about the “microstructure” of NREM sleep present sleep as an ever-changing fluctuating state intermingled with microarousals, strongly contradicting to this simple model.
Keywords
HypothalamusSleep regulationSleep switchFlip-flop modelNREM sleepWake-sleep transitionSleep microstructureThe first important step in understanding wake/sleep regulatory system in the brain was the discovery of the Viennese neurologist Baron Constantin von Economo in 1930. Based on clinicopathologic studies on victims of pandemic encephalitis lethargica, he observed that the prolonged sleepiness of one part of the victims is due to posterior hypothalamus and rostral midbrain injury. He has also observed that those affected people who suffered prolonged insomnia had lesions of the preoptic area and basal forebrain. Based on his observations, Economo assumed that the region of the hypothalamus near to the optic chiasm should contain sleep-promoting, while the posterior hypothalamus wake-promoting neurons (von Economo 1930). The key role of these two regions in sleep/wake regulation has been confirmed by animal experiments (Nauta 1946; Swett and Hobson 1968; McGinty and Sterman 1968).
Almost 20 years later, Moruzzi and Magoun (1949) discovered the ascending reticular arousal system (RAS), originating in the upper brainstem, fuelled by the sensory brainstem input pathways. During the subsequent years, the different chemical transmission branches of RAS system were explored in detail (Jones 2003).
The question how this powerful system of wakefulness is switched of when we fall asleep remained enigmatic for a long time. At the turn of the twentieth/twenty-first century, Saper et al. (2001) and Gallopin et al. (2000) showed clearly that the ventrolateral preoptic area (VLPO and extended VPLO) sends GABA-ergic and galaninergic inhibitory impulses to all the brainstem nuclei harboring the ascending pathways of the arousal systems and keeps firing throughout the whole NREM sleep, providing the substrate of the “sleep system” having opposite function to the “wake system.” Later, it turned out that the arousal systems also exert inhibitory effect on the “sleep-promoting” preoptic neurons (Saper et al. 2010).1
The discovery of the orexinergic system as lastly recognized special member of the arousal system provided further evidences for the mutually antagonistic system governing the actual balance between wakefulness and sleep (Nishino 2011). The area of basal forebrain has been thoroughly explored looking for sleep-promoting neuronal groups. It has been established by the c-Fos method that the ventrolateral preoptic nucleus (VLPO), a small circumscript area, exerts enduring neuronal activity during animal sleep. Further studies showed that in the preoptic region, a diffusely situated area (MnPN) has sleep-promoting activity too. Additional confirmation of the sleep-promoting role of this area came from neurotoxic destruction experiments of the VLPO and MnPN neurons (McGinty et al. 2004).
It has also been demonstrated that VLPO and the suprachiasmatic nucleus have synchronized activity, and both of them receive input from the retinal ganglionic cells. Therefore, circadian and visual information may modulate the VLPO activity that would be the substrate of circadian timing for the sleep process. Interesting tract-tracing and stimulation studies confirmed the presence of reciprocal inhibitory connection between the VLPO/MnPN neurons and the neurons of the ascending arousal system. These data serve as the substrate of the “sleep switch model” (Saper et al. 2001, 2010) based on the mutual inhibition between VLPO and the major arousal systems (Fig. 1.1).
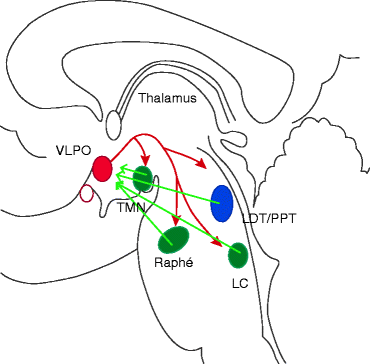

Fig. 1.1
A flip-flop switch model for wake-sleep regulation. Preoptic sleep-promoting neurons inhibit the arousal systems during sleep and are inhibited by the arousal systems during wake. As a result, the two mutually inhibitory systems form a flip-flop switch that is stable in either state but switches rapidly between them. Red arrows sleep promotion, green arrows wake promotion, VLPO ventrolateral preoptic area, LDT/PPT laterodorsal/pedunculopontine tegmental cells, LC locus coeruleus, TMN tuberomammillary nucleus (Modified from Saper et al. 2001)
When VLPO neurons fire during sleep they would inhibit the arousal system cell groups thus disinhibiting and reinforcing their own firing. Similarly when arousal neurons fire at high rate during wakefulness, they would inhibit the VLPO, thereby disinhibiting their own firing. This reciprocal relationship is similar to a type of circuit that electrical engineers call ‘flip-flop.’ The two halves of a flip-flop circuit, by each strongly inhibit the other; create a feedback loop that is bistable, with two possible stable patterns of firing and a tendency to avoid intermediate states (Saper et al. 2001).
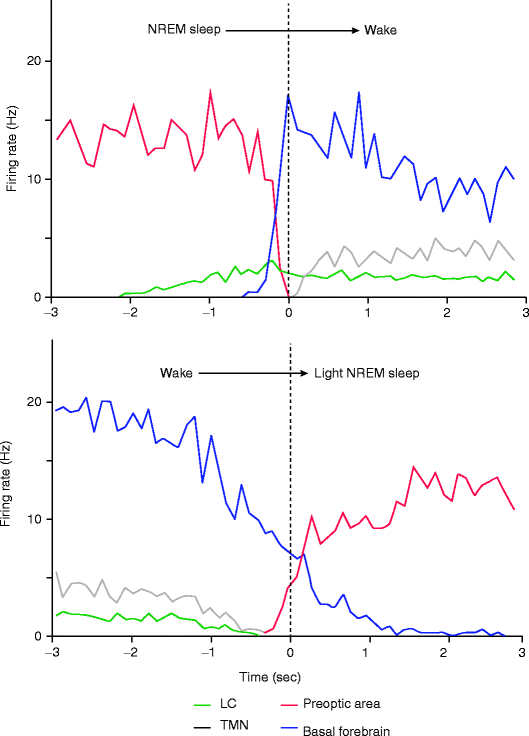
The experimental evidence for the sleep switch model of Saper is mainly based on the work of Takahashi and coworkers (2006, 2009, 2010). The Japanese group systematically detected the neuronal firing pattern of different sleep- and wake-promoting hypothalamic neuronal populations during falling asleep, sleeping, and awakening. Transitions from wake to sleep were characterized by the decrease of the firing rate of neurons of the wake-promoting and rising in the sleep-promoting neuronal network, and during awakening, an opposite dynamics of firing pattern was detectable (Fig. 1.2). These results confirm that these subcortical neuronal assemblies indeed have governing role in the EEG and behavioral changes during the sleep/wake cycle. However, the dynamics of falling asleep showed that the initiation of sleep is caused by the decreased activity of the wake-promoting neurons (disfacilitation) and not by the activity of sleep-promoting neurons (see also Steriade 2001). These results need confirmation and further research.