Developmental Characteristics of Sleep and Sleep Disorders in Children
Josephine Elia
Thornton B. A. Mason II
Sleep is a fascinating phenomenon, and the study of sleep and sleep disorders has expanded exponentially in the past two decades. Sleep medicine is an interdisciplinary field, and advances in our understanding of sleep medicine have been made by molecular biologists as well as behaviorists. Although the exact function of sleep remains undefined, disturbed sleep can impair daytime functioning and can be associated with major adverse health effects. Sleep differs across the lifespan, and this chapter highlights the types of sleep studies that can and have been used to explore normal variation in childhood and adolescence. There are many relevant sleep disorders, including disruptions in circadian patterns, parasomnias of non-rapid eye movement (NREM) and rapid eye movement (REM) sleep, sleep-disordered breathing, periodic limb movements/restless legs syndrome (RLS), and narcolepsy. As this chapter demonstrates, complex and intriguing relationships exist between attention deficit hyperactivity disorder (ADHD) and sleep, as well as between seizures and sleep. Furthermore, ADHD medications and anticonvulsants may also directly affect sleep. Importantly, there are bidirectional relationships between sleep and internalizing disorders such as depression, anxiety, and behavioral problems, all of which are summarized here.
Overview of Sleep
Broadly, sleep may be classified into REM sleep and NREM sleep. In turn, NREM sleep consists of sleep stages 1 through 4. Stages 1 and 2 are “lighter” sleep, from which a child may be awakened relatively easily. Stage 1 sleep may be intermixed with wakefulness as a child transitions to sleep. By electroencephalogram (EEG), stage 2 sleep is marked by Kcomplexes (high-amplitude deflections, with a negative component followed by a positive component) and sleep spindles (bursts of brief, 12- to 14-Hz activity); for both, the paroxysmal discharges stand out from the background EEG activity. The remaining NREM sleep stages (3 and 4) are sometimes referred to collectively as slow wave sleep or delta sleep. These stages are characterized by high-amplitude (<75 uV) slow waves (with frequencies less than 2 Hz), with stage 4 sleep having a higher prevalence of these waves (1). Slow wave sleep tends to predominate in the first half of the night (Fig. 6.1). With inadequate prior sleep or prolonged wakefulness, there is a greater pressure for slow wave sleep, so that the percentage of total sleep spent in slow wave sleep increases. REM sleep is particularly associated with dreaming, although dreaming can occur in other sleep stages. The
EEG in REM sleep resembles wakefulness. REM sleep has both phasic features (eye movements; brief, small amplitude limb movements) and tonic components (without these movements). Overall, motor tone is strikingly decreased in REM sleep. REM sleep predominates in the second half of the night in older children and adults (Fig. 6.1).
EEG in REM sleep resembles wakefulness. REM sleep has both phasic features (eye movements; brief, small amplitude limb movements) and tonic components (without these movements). Overall, motor tone is strikingly decreased in REM sleep. REM sleep predominates in the second half of the night in older children and adults (Fig. 6.1).
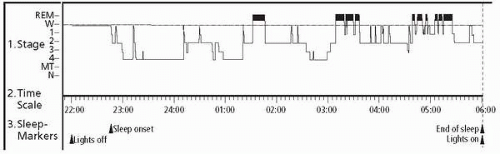 ▪ FIGURE 6.1 The hypnogram shows the pattern of wake (W), non-rapid eye movement (REM) sleep stages 1-4, and REM sleep during an overnight polysomnography recording in a sleep laboratory. Note the prolonged sleep latency of greater than 1 hour on the time scale (lights off to sleep onset), the preponderance of slow wave sleep (stage 4) in the first half of the study, and the REM sleep prominence in the second half of the study. |
Several types of studies have been developed to assess sleep. The gold standard for sleep analysis is overnight polysomnography. Polysomnography employs multiple leads to record important parameters simultaneously. By convention, four EEG leads are placed to measure central and occipital brain wave activity bilaterally. Two leads are placed on the outer canthus regions of the eyes to detect eye movements (blinking; REM sleep-associated movements). Airflow from the nose and mouth can be recorded by a thermistor that is sensitive to temperature differences between inhaled air (cooler) and exhaled air (warmer); nasal pressure transducers give a better indication, however, of the amount of air exchanged with breathing. In pediatrics, end-tidal carbon dioxide is frequently monitored by nasal cannula, and these data can be supplemented by transcutaneous carbon dioxide measurements if desired. Respiratory effort is measured by calibrated bands at the chest and abdomen. Electromyograms indicate muscle activity (movement) at several sites (chin region, legs). A single-lead electrocardiogram allows assessment of heart rate and screening for cardiac arrhythmias during sleep. A pulse oximeter is usually placed on a finger or toe to follow oxyhemoglobin saturations throughout the study. Currently, polysomnograms are recorded digitally, thereby allowing easy storage and facilitating analysis. Some polysomnography recordings are expanded with additional EEG leads for enhanced detection of epileptiform discharges or with a pH probe to detect gastroesophageal reflux. Concurrent video recordings are very helpful in detecting and characterizing a patient’s movements during a sleep study. The polysomnography data are scored into sleep stages based on established criteria (1). Similarly, the data are scored for sleep-disordered breathing (2,3), arousals/awakenings, and leg movements (4).
When excessive daytime sleepiness is a concern, a multiple sleep latency test (MSLT) is sometimes performed on the day following an overnight polysomnogram. The set-up for an MSLT includes some but not all of the leads from an overnight study. Beginning 1½ to 2 hours after awakening from the overnight study, the patient will have a series of nap opportunities every 2 hours, with four to five naps total. The duration of the nap opportunities is standardized, and for each nap there is a determination of whether the child sleeps or not and, if so, the latency to sleep and the presence of REM sleep are noted. By averaging the results of all the naps, a mean sleep latency is determined (5).
Actigraphy has been used in both research and clinical settings for the assessment of sleep disorders in children and adults. Typically worn on the wrist of the nondominant hand, an actigraph resembles a watch and records the frequency and magnitude (acceleration/deceleration) of movement, which can in turn be used to generate rest-activity patterns; these patterns have been shown to be valid indications of sleep-wakefulness, respectively (6). Actigraphy recording over 1 to 2 weeks should allow for variation in weekday versus weekend schedules and, when paired with a concurrent sleep diary, can reflect a patient’s typical (at home) sleep pattern. Some of the parameters that can be determined by routine actigraphy include sleep latency, total sleep time, the number of defined night wakings, the total duration of night wakings, and sleep efficiency (time spent asleep divided by total time in bed) (7,8).
Parents and older children can be asked to give details about their sleep and wake patterns through a sleep diary. The parents can indicate when a child is in bed ready for sleep; when the child actually falls asleep; the timing, duration, and number of night-time awakenings; the morning rise time; and the presence of any naps. Sleep diaries can effectively highlight insufficient sleep, weekday versus weekend variation, and erratic sleep patterns. A number of sleep questionnaires have also been developed to assess pediatric sleep and may serve as other valuable means of gathering clinical data (e.g., see references 9, 10, 11).
Sleep Duration Across Childhood and Adolescence
Total sleep duration is typically longest during infancy and decreases consistently from childhood through adolescence: total sleep duration is 14.2 (± 1.9) hours in 6-month-olds, 11 (± 0.8) in 6-year-olds, 9.3 (± 0.6) in 12-year-olds, and 8.1 (± 0.7) in 16-year-olds (12). Infants’ and young children’s naptime is included in calculating total sleep duration. Newborns are not entrained to light-dark cycles and spend the majority of each 24-hour period asleep, with a total of about 7 sleep and wake periods in 24 hours. Sleep is consolidated at 4 months of age into about three or four sleep periods, with two thirds of sleep occurring at night. With increasing age, the number of naps decreases. By around 18 months of age, children transition to a single nap per day By age 3 years, only about half of children take a daytime nap, and naps chiefly disappear by school age (12). Indeed, it is abnormal for a prepubescent school-aged child to exhibit daytime sleepiness.
Regarding sleep architecture, in infants less than a year of age there is frequently a transition from wake to REM (active) sleep, and then to NREM (quiet) sleep. The incidence of these direct wake-to-REM transitions decreases between 3 and 9 months of age, but REM latency (the time from sleep onset to REM onset) is still very short (13). There is a relative prolongation of REM latency during childhood. Indeed, Montgomery-Downs et al. (14) studied normal children ages 3 to 8 years and reported that REM latency increased linearly across age in this window, with significant changes from ages 4 to 5 years and 5 to 6 years. The number of REM/NREM cycles also changes with development. Children aged 3 to 5 years have been found to have six cycles (mode), whereas children 6 to 7 years have five cycles. For both age groups, a significant linear trend has been reported for increased minutes of REM sleep across the night within cycles (14).
Behavioral Insomnia of Childhood
As reviewed by Mindell et al. (15), behavioral insomnia in children frequently presents with bedtime resistance or night-time awakenings or both. It occurs in 20% to 30% of young children. Mild and transient symptoms are common and constitute a disorder only
if they are persistent and impair the child and/or the family. The two broad categories include sleep-onset association disorder and limit-setting disorder. Bedtime behavioral insomnia is often associated with children whose temperaments may be more irritable or require more adult intervention to calm down (16,17).
if they are persistent and impair the child and/or the family. The two broad categories include sleep-onset association disorder and limit-setting disorder. Bedtime behavioral insomnia is often associated with children whose temperaments may be more irritable or require more adult intervention to calm down (16,17).
Sleep-onset association disorder refers to specific conditions that facilitate a child’s falling asleep but when absent may result in prolonged sleep latency or nightly awakenings. Examples include positive associations that are controlled by the child (e.g., thumb sucking, cuddling a stuffed animal) and negative associations that are controlled by others (e.g., rocking, feeding, chauffeuring by parent). Given that children normally have brief arousals during the night (two to six times) (18), prolonged awakenings can result when the sleep association that they cannot control is not immediately available and can be disruptive when accompanied by crying and getting out of bed (19). Sleep association disorders affect infants and toddlers (ages 6 months to 3 years) and are not considered before 6 months of age, before an infant has reached a neurodevelopmental stage at which adequate sleep consolidation and regulation allow sleep for an extended amount of time (19). Sleep-onset association disorder can also affect the older child who may, for example, require a parent to lie in the same bed in order to fall asleep; when the child wakes during the night and the parent is no longer in the bed, the child may be unable to return to sleep.
Limit-setting sleep disorder refers to bedtime delay tactics (e.g., requesting additional bedtime stories, asking for a drink of water, posing multiple questions, debating), refusal to go to bed, or refusal to stay in bed. It occurs predominantly when parents do not set and enforce consistent limits. The delays result in shorter total sleep time but not in diminished sleep quality. Combined type sleep-onset association and limit-setting behavioral insomnia may also occur simultaneously in some children.
Effective treatments for behavioral insomnia include parental education and behavioral management techniques, such as extinction, graduated extinction, and positive routines. As reviewed by Mindell et al. (15) extinction procedures necessitate that the child is in bed at a designated time and parents refuse to respond to any requests or crying for as many nights as it may take to stop the behavior. A modified version in which parent(s) stay in the child’s room but ignore the behavior may be more acceptable to some parents. Graduated extinction involves increasing the time interval between successive checks during which time the parent comforts the child for 15 to 30 seconds. Positive routines initially focus on developing a bedtime routine associated with quiet activities enjoyable to the child. Bedtime is delayed intentionally so that sleep cues become associated with positive experience, and once this is established, bedtime is gradually moved to an earlier time. The child may respond to small rewards for falling asleep independently. Behavioral treatments are preferred to pharmacotherapy.
Delayed Sleep Phase Syndrome
A circadian rhythm sleep disorder, delayed sleep phase syndrome type (DSPS) is characterized by difficulty with both falling asleep and awakening at conventional times (20). Sleep itself was thought to be normal (21); however, a controlled study has shown increases in stage 1 and 2 of sleep and decreases in slow wave sleep (22). The cause is complex: although cultural factors play a role, delays in circadian rhythms, including body temperature and melatonin rhythms, point to biological intrinsic factors in adults (23,24) as well as adolescents (25). Circadian gene variants have also been linked to DSPS, including Per3 (26), 3111 Clock (27), arylalkalamine N-acetyltransferase (28), and HDLA-DR1 (29).
A shift toward delayed sleep onset emerges as children enter adolescence (25,30), when offset of melatonin secretion has been shown to correlate with age and Tanner stage (31). This pubertal associated phase delay in circadian rhythms appears to continue in adolescence; melatonin secretion was actually delayed in 10th graders who started their day 65 minutes earlier, suggesting an impairment in adjusting sleep patterns to optimize sleep (32). DSPS with an early rise time can result in significant sleep deprivation that can impair daily functioning, worsen school performance, and increase moodiness (11). In young adults, car crashes are a significant risk (33).
Effective treatments include chronotherapy, exposure to early morning light, and exogenous melatonin. Chronotherapy involves delaying sleep onset for approximately 3 hours every day until sleep occurs at the designated conventional time (34). This treatment has practical limitations and the potential risk of inducing a non-24-hour sleep-wake cycle (35). An alternative to delaying the sleep-wake phase is advancing it with light-dark exposure and medication. Controlled light-dark exposure can shift circadian phase in adolescents (31). Exposure to light therapy in the morning with evening light restriction may restore a conventional time schedule (36, 37, 38). Melatonin administered in the late afternoon or early evening also has been found effective in advancing the circadian sleep clock (39).
Case One
During the parent-teacher conference, J.J.’s parents learn that their 14-year-old son is dozing off during the first and second periods. Bedtime on weeknights is 10:00 p.m., but frequently he is emailing friends and not falling asleep until 11 to 12 midnight. Aside from English (second period class) he has excellent grades, is well liked, and is cooperative at home. Therefore, his parents feel that he has earned the right to stay up later on weekends. He also sleeps later in the morning, allowing him to catch up on any missed sleep. What steps should be taken to improve this child’s sleep?
Discussion:
Weekend schedules that permit later bedtime and awakening can exacerbate a young adult’s natural tendency to delay sleep onset. Computers serve as light sources, further delaying sleep onset. Minimizing these could be helpful. In addition, advocating for school days that start later for adolescents is also important.
Arousal Disorder Parasomnias
An important subset of pediatric parasomnias includes the disorders of arousal. These parasomnias may be considered part of a continuum, as they share overlapping features: sleepwalking, confusional arousals, and sleep terrors. Although most often occurring in slow wave sleep (stages 3 and 4 of NREM sleep), these parasomnias can also arise from stage 2 NREM sleep (40). Common among these disorders include incomplete transition from slow wave sleep, automatic behavior, altered perception of the environment, and variable degrees of amnesia for the event. The child’s sleep stage transition from slow wave sleep is abnormal, often when shifting into lighter NREM sleep (e.g., stage 2) just prior to
the first REM sleep episode. The patient in a sense becomes “stuck” between deep sleep and wakefulness (41). The EEG during these episodes demonstrates an admixture of theta, delta, and alpha frequencies. The disorder of arousal parasomnias are all more frequent in childhood than in adolescence or adulthood. Prevalence estimates in childhood for sleep terrors range from 1% to 6%; for sleepwalking, up to 17% with a peak at 8 to 12 years; and for confusional arousals, up to 17.3% (42).
the first REM sleep episode. The patient in a sense becomes “stuck” between deep sleep and wakefulness (41). The EEG during these episodes demonstrates an admixture of theta, delta, and alpha frequencies. The disorder of arousal parasomnias are all more frequent in childhood than in adolescence or adulthood. Prevalence estimates in childhood for sleep terrors range from 1% to 6%; for sleepwalking, up to 17% with a peak at 8 to 12 years; and for confusional arousals, up to 17.3% (42).
Sleepwalking (somnambulism) in childhood shares features with that in adults. Sleepwalking may be either calm or agitated, with varying degrees of complexity and duration (43). The frequency of sleepwalking may be underestimated because of episodes that are unobserved or unremembered (44). Children with somnambulism are usually calm and do not demonstrate fear. They may walk into a parent’s room, bathroom, or different parts of the house. Children with sleepwalking are at risk for injury. They may climb through windows, wander in bathrooms, attempt to walk downstairs, and sometimes leave the house. Injuries may include trauma from falls, lacerations from broken windows or patio glass doors, and even hypothermia from exposure.
Confusional arousals, which occur mainly in infants and toddlers, have more associated agitation than what would be expected with sleepwalking. A typical episode may begin with movements and moaning, and then evolves to confused and agitated behavior with calling out, crying, or thrashing (45). Attempts to wake the child fully are unsuccessful. The child appears confused, with eyes open or closed, and is very agitated or even combative. Physical injury is rarely seen (46). The child resists the parents’ efforts at consolation, and more forceful attempts to intervene may result in increased resistance and further agitation. A confusional arousal episode may last 5 to 15 minutes (although sometimes longer) before the child calms and returns to a restful sleep.
Sleep terrors are dramatic partial arousals from slow wave sleep. The child may sit up suddenly and scream, with an intense, blood-curdling “battle cry.” The episode is a fightflight phenomenon. Autonomic activation is present, with mydriasis, diaphoresis, and tachycardia (47). Respiratory tidal volume is increased, and there is an intense look of fear on the face. Moreover, there is a “curious paradox” of endogenous arousal coexistent with external unarousability (48). With sleep terrors, children may report indistinct recollections of threats (monsters, spiders, snakes) from which they have to defend themselves (45,49). The differential diagnosis of sleep terrors includes nightmares, nocturnal panic attacks, epileptic events, and cluster headaches.
Multiple factors may influence arousal parasomnias. Age is an important issue, as many parasomnias are much more likely to occur in childhood than later in life. Other contributing factors include the homeostatic drive to sleep (with more frequent or more severe parasomnia episodes being associated with prolonged sleep deprivation), medications (e.g., neuroleptics, sedative hypnotics, stimulants, and antihistamines), a noisy or stimulating sleep environment, fever, stress, and intrinsic sleep disorders [such as obstructive sleep apnea and periodic limb movements in sleep (PLMS)] (50). In children, sleep-disordered breathing or PLMS-RLS may trigger sleep walking or sleep terrors, as these parasomnias have been reported to disappear after obstructive sleep apnea or PLMS-RLS treatment (49). In childhood, psychopathology is thought to be extremely rare as an influencing factor for arousal parasomnias (51). Several studies support a genetic predisposition (52,53).
Treatment of disorders of arousal in childhood includes reassuring parents that parasomnias are common and can be managed effectively. The parents should be counseled, when appropriate, on instituting important safety measures, that is, placing the mattress on the floor, securing the windows and outside doors, covering the windows with heavy curtains, and using alarm systems and bells to alert parents if the child leaves the room. Other nonpharmacological treatments include maintaining a sleep diary, which will foster
routine notation of sleep times and may help to reinforce the principle of minimizing sleep deprivation, and eliminating caffeine-containing beverages. Medications are indicated for those rare, protracted cases with no associated sleep disorder, with frequent parasomnias, and with a threat of injury to the patient or others. Benzodiazepines and tricyclic antidepressants have been used successfully (54).
routine notation of sleep times and may help to reinforce the principle of minimizing sleep deprivation, and eliminating caffeine-containing beverages. Medications are indicated for those rare, protracted cases with no associated sleep disorder, with frequent parasomnias, and with a threat of injury to the patient or others. Benzodiazepines and tricyclic antidepressants have been used successfully (54).
Case Two
A mother brings her 4-year-old daughter to the office because of frequent “nightmares.” The girl falls asleep between 9:30 and 10 p.m. nightly and two to three times per week awakens suddenly between 11 p.m. and midnight. Her daughter screams with amazing intensity and then appears sweaty and moderately agitated, often pacing in her room. She reports that the girl does not respond to her (“looks right through her”), and her mother cannot do anything to stop an episode. In total, an episode can last 10 to 30 minutes. On the mornings following, her daughter has no recollection of the events and appears to be her normal, well-rested self. What is the likely diagnosis and the recommended treatment?
Discussion:
These impressively intense events are consistent with sleep terrors. Key features include their typical onset 90 minutes to 2 hours after sleep onset, prominent agitation, the parent’s inability to calm the child, and the girl’s amnesia for the event. Her mother should first be reassured that sleep terrors are akin to sleep walking, are relatively common, are not routinely associated with psychiatric disorders, and usually disappear by adolescence. Sleep deprivation makes sleep terrors (and other arousal parasomnias) more likely, so the initial management should be focused on increasing total sleep duration. For this child, the bedtime should be gradually advanced to 8 p.m.; this will likely result in fewer, milder sleep terror episodes, if not eliminating them altogether. Medication is not indicated at this time.
Nightmares
Nightmares are characterized by terrifying dreams followed by awakening with vivid recall of the content and difficulty returning to sleep. An occasional nightmare occurs in more than 55% of children and adolescents, whereas 5% experience these several times per week (55). Nightmares occur more frequently in girls than in boys (56). They typically emerge after a very frightening or traumatic experience or a severe medical illness (57). Medications that can affect the central nervous system can also precipitate nightmares, but this occurs primarily in adults (58).
Children with nightmares have higher rates of sleep terrors, sleep walking, anxiety at bedtime, daytime somnolence (56), and snoring (59). Nightmares are also associated with daytime behavioral and emotional difficulties (55). In a 14-month follow-up study of 5- to 8-year-olds, nightmares persisted in 30% but parents did not express a need to seek remedies (56).
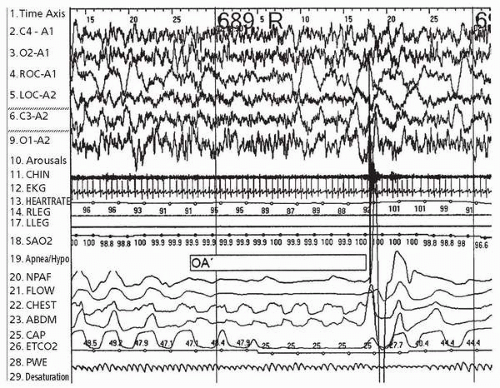
Sleep-disordered Breathing
Obstructive sleep apnea (OSA) is a term used to describe airway obstruction that results in decreased air exchange during sleep. Overnight polysomnography can demonstrate obstructive apneas (little or no air movement despite ongoing and sometimes increased respiratory effort) and obstructive hypopneas (shallow breathing), often in the setting of loud snoring and paradoxical breathing (thoracoabdominal asynchrony) (Fig. 6.2). In children, obstructive events tend to cluster during REM sleep, with breathing relatively unaffected often during NREM sleep. Moreover, despite moderate to severe obstructive sleep apnea in some children, sleep architecture tends to be preserved. Estimates of the prevalence of obstructive sleep apnea in children vary, based on the sensitivity and specificity of responses to questionnaires, a range of different sleep study techniques (not limited to overnight polysomnography), and the particular criteria applied for establishing obstructive sleep apnea (60). Snoring is extremely frequent in children and is associated with obstructive sleep apnea, as snoring represents a symptom of increased upper airway resistance. Snoring is found in 18% to 20% of infants, 7% to 13% of children ages 2 to 8 years, and 3% to 5% of older children (61).
In general, the prevalence of pediatric obstructive sleep apnea is approximately 2% (62). Obstructive sleep apnea in childhood is important because it may result in growth failure, cor pulmonale, developmental delay, and poor school performance (63,64).
Adenotonsillar hypertrophy plays a major contributory role; others include craniofacial structure, obesity, and neural control mechanisms of the upper airway (61,65, 66, 67). Genetics may also be important, as studies in adults have demonstrated a familial aggregation of sleep apnea (68, 69, 70), and a few studies have suggested such aggregation exists for pediatric sleep apnea (71,72).
Adenotonsillar hypertrophy plays a major contributory role; others include craniofacial structure, obesity, and neural control mechanisms of the upper airway (61,65, 66, 67). Genetics may also be important, as studies in adults have demonstrated a familial aggregation of sleep apnea (68, 69, 70), and a few studies have suggested such aggregation exists for pediatric sleep apnea (71,72).
Obstructive sleep apnea can be treated effectively (62). Because of the association with enlarged tonsils and adenoids, adenotonsillectomy has been recommended as first-line treatment, particularly for children in the 2-to 6-year age range. Weight loss, although helpful in decreasing lateral airway narrowing in obesity, is difficult to achieve and therefore may only complement other treatments. Continuous positive airway pressure (CPAP) therapy essentially uses a column of air to splint the airway open; bilevel positive airway pressure (BLPAP) allows lower pressures during expiration, and a backup or timed delivery of breaths if needed.
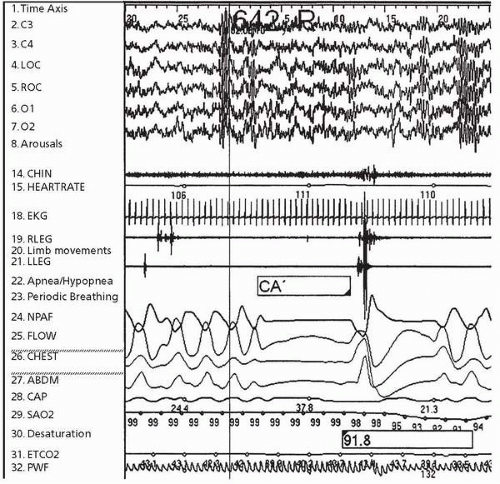
Central sleep apnea, which is fundamentally different from obstructive sleep apnea, is characterized by a diminished to absent drive to breathe (Fig. 6.3). Central apnea,
sometimes associated with prematurity, improves with advancing postconceptual age. In some patients, central apnea is physiologically driven, such as during periods of sleep-wake transition or when sleeping at high altitudes. Whereas respiratory drive during wakefulness is under the control of cortical regions, respiratory drive during sleep is predominantly under brainstem control. Therefore, pathological brainstem processes, such as a Chiari I malformation, can be associated with central sleep apnea and central hypoventilation (marked by an increase in arterial carbon dioxide tension). Central hypoventilation in children may also occur secondary to hydrocephalus, hypoxic-ischemic encephalopathy, achondroplasia with stenosis of the foramen magnum, trauma, brainstem tumors, encephalitis, familial dysautonomia, and mitochondrial disorders (73). Rarely, central sleep apnea can occur in the setting of congenital central hypoventilation syndrome.
sometimes associated with prematurity, improves with advancing postconceptual age. In some patients, central apnea is physiologically driven, such as during periods of sleep-wake transition or when sleeping at high altitudes. Whereas respiratory drive during wakefulness is under the control of cortical regions, respiratory drive during sleep is predominantly under brainstem control. Therefore, pathological brainstem processes, such as a Chiari I malformation, can be associated with central sleep apnea and central hypoventilation (marked by an increase in arterial carbon dioxide tension). Central hypoventilation in children may also occur secondary to hydrocephalus, hypoxic-ischemic encephalopathy, achondroplasia with stenosis of the foramen magnum, trauma, brainstem tumors, encephalitis, familial dysautonomia, and mitochondrial disorders (73). Rarely, central sleep apnea can occur in the setting of congenital central hypoventilation syndrome.
Whenever feasible, the cause of secondary hypoventilation should be treated (e.g., placement of a ventriculoperitoneal shunt for hydrocephalus). The mainstay of management for children without a treatable cause should be chronic ventilatory support. Although central apnea in preterm infants can improve with caffeine and other respiratory stimulants, pharmacotherapy has not been successful in other forms of central hypoventilation. Noninvasive ventilation (e.g., BLPAP) has been used successfully, but must be monitored carefully to ensure adequate ventilatory control. Tracheostomy is usually required in patients with congenital central hypoventilation syndrome, and diaphragmatic pacers may allow increased mobility for those who require ventilatory support while both awake and asleep (73).
Case Three
A 6-year-old boy presents with loud snoring, gasping in sleep, and pauses in breathing according to his parents’ report. His academic performance in first grade has been below average. He is irritable at times but does not nap or appear sleepy. On examination, he breathes primarily through his mouth and has moderately hypertrophied palatine tonsils. If obstructive sleep apnea is suspected, what should be done next?
Discussion:
An overnight polysomnogram should be performed to evaluate for the presence and severity of obstructive sleep apnea. The polysomnogram provides a quantitative assessment, including the number of obstructive respiratory events per hour (an apnea-hypopnea index) and parameters of respiratory gas exchange (e.g., the oxygen saturation nadir, and the mean and peak end-tidal CO2 values). In childhood, most obstructive respiratory events occur in REM sleep (when breathing patterns are irregular and muscle tone is low); accordingly, oxygen desaturations typically also cluster in REM sleep. If the polysomnogram confirms obstructive sleep apnea, a referral to an ear, nose, throat (ENT) physician would be reasonable; the tonsils are large, and this boy’s prominent mouth breathing suggests adenoid hypertrophy. In most cases, adenotonsillectomy will resolve the upper airway obstruction and may also improve daytime behavior, including school performance.
Seizures and Sleep
Seizures can occur during sleep as well as during wakefulness. Indeed, some patients have seizures more frequently, or even exclusively, during sleep. There are several seizure types in which events are prominent in sleep, including benign rolandic epilepsy, electrical status epilepticus of sleep, and nocturnal frontal lobe epilepsy. Benign rolandic epilepsy begins in childhood and typically resolves in adolescence; seizures occur in the evening hours, often in the wake-sleep transition period. The classic clinical presentation of benign rolandic epilepsy includes brief, partial, hemifacial motor seizures, with or without somatosensory symptoms (74), also often involving jerking of the ipsilateral the upper extremity; concurrent drooling and speech arrest are often seen, with preserved alertness (75). More rarely, patients may have secondarily generalized tonic-clonic seizures (74). The EEG demonstrates epileptiform activity (sharp waves, often followed by slow waves) in centrotemporal, midtemporal, frontocentral, centroparietal, or centro-occipital locations (74). With a typical presentation, anticonvulsant treatment is not always required.
Electrical status epilepticus of sleep is a dramatic finding: a normal EEG pattern during wakefulness changes to a generalized spike-wave pattern during sleep. The diffuse epileptiform activity is nearly persistent (e.g., often more than 85% of the NREM sleep record). Electrical status epilepticus of sleep may be associated with cognitive and language dysfunction, although a precise temporal relationship between the appearance or presence of the EEG abnormality and clinical features has not been established. In a given patient, electrical status epilepticus of sleep may wax and wane in severity but can be seen over years (76). No single therapy is recognized as effective, although there are reports of treatment attempted with intravenous immunoglobulin, prednisone, diazepam, and/or levitiracetam (76).
Autosomal dominant nocturnal frontal lobe epilepsy is a genetic epilepsy syndrome that manifests as abnormal activity during sleep. One subtype is paroxysmal arousals, in which patients have a brief (<20 second) stereotyped sequence of behavior (including eye opening, rising from the bed with abnormal limb posturing, and showing an expression of apparent fear or surprise). Nocturnal paroxysmal dystonia is marked by dystonic posturing of the limbs, trunk, or head; there may be associated bizarre or violent (ballistic) movements. The duration is often less than 2 minutes. Episodic nocturnal wandering is more rare; these events include paroxysmal ambulation during sleep, possibly accompanied by screaming or agitated behavior, and lasting 1 to 3 minutes (77,78). Nocturnal frontal lobe epilepsy has a mean age of onset of 10 to 12 years. For any of these frontal lobe epilepsy manifestations, it is difficult to capture ictal discharges by EEG, so an empiric trial with carbamazepine (which is especially effective) may be warranted if the events are clinically suspicious for seizures. Differentiating nocturnal frontal lobe epilepsy from arousal parasomnias (such as confusional arousals or agitated sleepwalking) can be challenging, but features supporting seizures include a slightly later onset of events (adolescence) with stability over time (i.e., persistence with advancing age), relatively short duration of events, stereotyped features particularly at onset, and the presence of dystonic posturing (79). Genetic studies in families have shown that autosomal dominant frontal lobe epilepsy is linked to mutations in nicotinic acetylcholine receptor subunits (CHRNA4 and CHRNB2) (80).
The relationships between sleep and epilepsy extend beyond these associations with specific epilepsy syndromes. Interictal epileptiform discharges are increased by sleep and sleep deprivation. Seizure frequency can be exacerbated by chronic sleep deprivation, and case series have supported improved seizure control in patients following OSA treatment (81,82). The greatest frequency for focal discharges is in slow wave sleep, where neuronal
synchrony is promoted. On the other hand, epileptiform discharges decrease dramatically during REM sleep (83). If a complex partial seizure or secondarily generalized seizure occurs during sleep, subsequent sleep is less efficient, with altered sleep architecture, including decreased REM sleep and, in some cases, less slow wave sleep. (84). Consequently, excessive daytime sleepiness in patients with epilepsy can result from seizure burden as well as anticonvulsant effects and any concurrent sleep disorders. Patients with tic disorder and Tourette syndrome also have decreased sleep efficiency, and similarly this disturbed sleep may affect daytime behavior (85,86).
synchrony is promoted. On the other hand, epileptiform discharges decrease dramatically during REM sleep (83). If a complex partial seizure or secondarily generalized seizure occurs during sleep, subsequent sleep is less efficient, with altered sleep architecture, including decreased REM sleep and, in some cases, less slow wave sleep. (84). Consequently, excessive daytime sleepiness in patients with epilepsy can result from seizure burden as well as anticonvulsant effects and any concurrent sleep disorders. Patients with tic disorder and Tourette syndrome also have decreased sleep efficiency, and similarly this disturbed sleep may affect daytime behavior (85,86).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree