Figure 50.1. Congophilic deposits characteristic of amyloid angiopathy.
Other risk factors for ICH such as caffeine consumption and illicit drugs use, obesity and antiplatelet therapy are areas of recent analysis. Anticoagulants usually are associated with large hemorrhages and high mortality.
50.5 Clinical Diagnosis
Clinical manifestations of ICH depend on the location of the hematoma, its initial volume and growth, aperture to the ventricular system, as well as mass effect and midline deviation. Patients with ICH usually present with severe neurological impairment after the onset and can worsen in the first hours. Headache, altered consciousness, motor or sensory deficit and seizures are frequent in supratentorial ICH, whereas headache, dizziness, vomits, ataxia, ophthalmoparesis and deterioration of consciousness with or without motor signs are proper to infratentorial ICH. Because its clinical presentation can also occur in ischemic CVD, neuroimaging studies are essential to differentiate between these possibilities.
50.6 Neuroimaging Diagnosis
50.6.1 Computed Tomography
Simple phase CT easily distinguishes ICH from cerebral infarct (Figure 50.2). It practically detects 100% of hematomas, except when <1 cm, located in the posterior fossa or in those patients with severe anemia (hematocrit <20) [16].
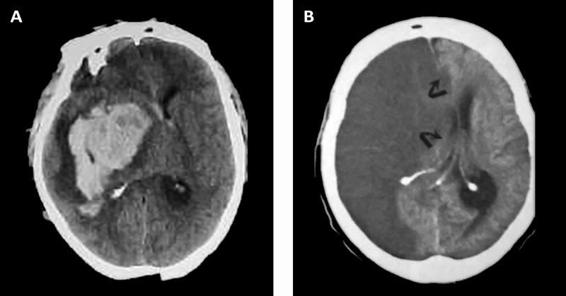
Figure 50.2. (A) CT scan with massive right ganglia hemorrhage. (B) Right hemispheric infarct. Both images show pronounced mass effect and midline displacement.
Contrast-enhanced CT is not necessary in acute stage ICH and its indication should be reserved for specific cases depending on hematoma location, the presence of edema and other features suggestive of vasculitis, rupture of CVM or intra-tumor bleeding [1,16]. Besides its utility in determining hematoma size and location, CT also allows the follow-up of complications, including cerebral herniation, midline displacement, cistern and brainstem compression, opening to the ventricular system, hydrocephalus, rebleeding, peri-hematoma and delayed edema.
Location
Topography considers the epicentre as the site where the hematoma originates. For a more precise location, we must clarify whether the ICH is supra- or infratentorial. In supratentorial ICH, a lobar hematoma (frontal, temporal, parietal, occipital); basal ganglia (putamen, caudate nucleus, thalamus, striato-insular) or intraventricular location must be specified. Infratentorial ICH is localized in the brainstem (midbrain, pons, medulla) or the cerebellum (Figure 50.3).
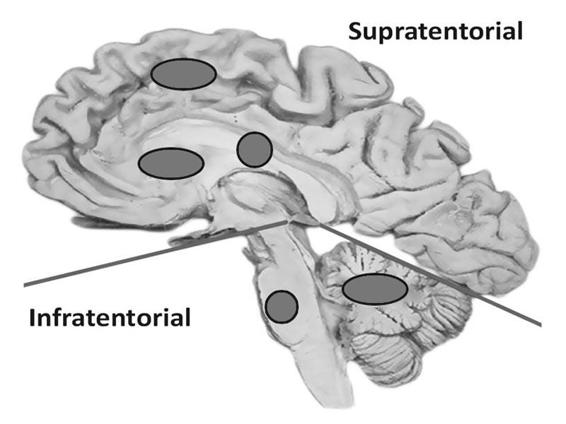
Figure 50.3. Supra- and infratentorial locations of intracerebral hemorrhage.
Ventricular Opening
Irruption of blood into the ventricular system has been associated with poor prognosis, which is why it is important to establish its presence or absence, to identify the ventricles involved, and determine its volume if possible [17] (Figure 50.4).
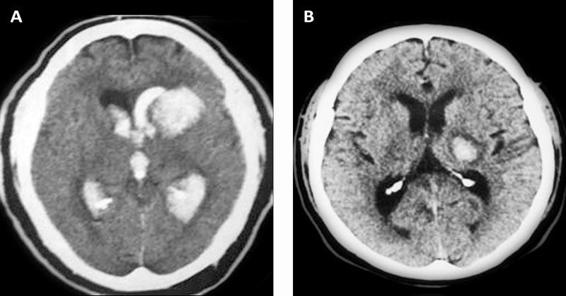
Figure 50.4. (A) Intracerebral hemorrhage with and (B) without ventricular aperture.
Intraventricular hemorrhage volume can be measured with the use of sophisticated CT scan software; however, because of its infrequent use, it is recommended to calculate the Graeb´s index [17]. Mild=1-4; moderate=5-8; severe=9-12 (Table 50.1).
Score | |
Lateral ventricles (each ventricle apart) | |
Blood traces in the ventricle | 1 |
Blood fills less than half of the ventricle | 2 |
Blood fills more than half of the ventricle | 3 |
Lateral ventricle is full and expanded | 4 |
Third and fourth ventricle (each ventricle apart) | |
Blood is present in the ventricle | 1 |
Ventricle is full and expanded | 2 |
Table 50.1. Graeb’s index to calculate intraventricular hemorrhage extent.
Volume
Hematoma volume is an important predictor of mortality, making its measurement necessary. As in intraventricular hemorrhage, the use of CT software allows the quantification of hematoma volume; however, since institutions may lack CT scan tools, a knowledge of alternative methods to calculate hematoma volume is needed. The most useful and common method is the ABC/2 formula (considering ICH as an ellipsoid or a football) [18,19]. The major diameter of hemorrhage (A) is multiplied by its perpendicular major diameter (B), times the number of sections (in cm) in which the hemorrhage appears on the CT scan (C), and the final product is divided by two. To calculate the value of C, the CT slice showing the largest hematoma is taken as a reference (100%) and the smallest as 25%, because the size of the hematoma will appear different on the sequence of CT scans; if a subsequent section of the reference slice shows a hematoma half its size, the value is corrected by giving this section 0.5 cm (Figure 50.5).
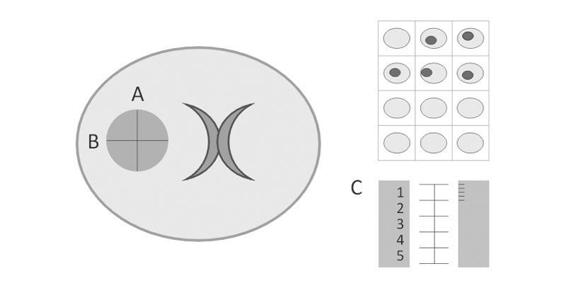
Figure 50.5. Calculation of hematoma volume with the ABC/2 formula.
Density and Shape
A recent analysis of the treatment of ICH with activated Factor VII proposed that a strong predictor for hematoma expansion is irregularity in the shapes and edges of the hemorrhage, as well as heterogeneity in its density [20]. This issue will be discussed later.
Other Tomographic Characteristics of ICH
Imaging findings vary depending on the evolution of the hemorrhage over time. In the hyperacute stage (first 4 hours), the extravasated blood has not yet formed a clot, producing an irregular hyperdense collection within the cerebral parenchyma. The presence of a blood-fluid level suggests an ICH in a very early phase, hemorrhage within a pre-existing cavity, or a hemorrhage due to coagulopathy or fibrinolytic therapy. In the acute phase (5 to 72 hours), a pre-existing hematoma becomes dense due to clot formation [21].
50.6.2 Computed Angiotomography (CTA)
CTA is an alternative non-invasive tool to angiography and magnetic resonance angiography (MRA), with the disadvantage that it requires contrast material and prolonged processing time. Nevertheless, a recent finding during the procedure (within the first 3 hours of ICH) termed “spot sign”, has been proposed as a strong predictor of hematoma expansion. The sign consists of a small “spot” or “point” inside the hematoma and suggests active bleeding from Charcot microaneurysms or from vessels with vascular damage [22].
50.6.3 Angiography
Angiography should be reserved for patients without a clear cause of ICH, especially in young normotensive patients, subjects with hemorrhage opened to the subarachnoid space, in those with prominent vessel structure as evidenced by CT or MR, when a vasculitis is suspected or in cases of sympathomimetic drug use [16,23,24]. Angiography is also recommended in patients with primary intraventricular hemorrhage and in those with perisylvian sulcal or fronto-orbital hemorrhage to dismiss the presence of aneurysms [16,23].
50.6.4 Magnetic Resonance and Magnetic Resonance Angiography (MRI, MRA)
These imaging studies are recommended in aged patients with absolute or relative contraindications to angiography due to blood dyscrasias, anticoagulant use, absent femoral pulse, severe atherosclerosis, as well as contraindication of the use of contrast material in patients with renal failure or multiple myeloma [16,23].
MR provides more information than CT about the hematoma borders, peri-hematoma edema and mass effect over other structures. It also helps to evaluate the damage associated with the hematoma and to define its possible mechanism, for example: arteriovenous malformation (heterogeneous image with empty signals and calcifications), intratumoral bleeding or cavernous angioma [16,23-27]. The disadvantages of MR include long procedural time, contraindication in persons with metal prosthesis, pacemaker wearers, or in those under mechanical respiration or multimonitoring.
MR with gradient-echo (also T2*) has proved an important tool in the study of quiet focal damage that corresponds histopathologically to hemosiderin deposits, termed micro-hemorrhages or microbleeds. Lobar microbleeds have been related to amyloid angiopathy, whereas ganglionar microbleeds have been associated with hypertension [28].
50.6.5 Histopathologic Study
In all patients with hematoma evacuation or autopsy, it is mandatory to confirm the etiologic diagnosis. The sample must include the wall of hematoma and blood vessels. Congo red tissue staining is necessary in elderly subjects to confirm amyloid ICH [29].
50.7 Etiology of Intracerebral Hemorrhage
Hypertension is the main cause of ICH, explaining 60-80% of cases. Hypertension places arterioles (100 to 300 microns in diameter) under constant mechanical stress, leading to hyperplasia of smooth muscle cells which results in atherosclerosis [1,12] (Figure 50.6).
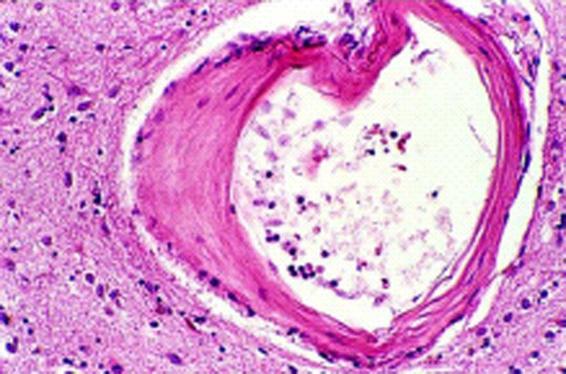
Figure 50.6. Arteriole in which the muscle wall has been replaced by collagen.
Over time, the muscle cells die and are replaced by collagen tissue (lipohyalinosis), leading to ectasia and occlusion (principal substrate of infarcts) and the formation of Charcot-Bouchard microaneurysms (considered today as the origin of ICH) (Figure 50.7) [1,12].
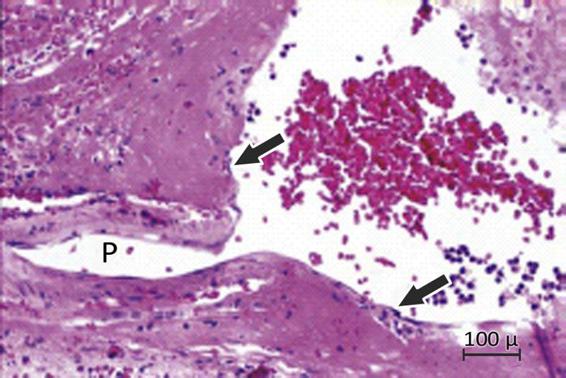
Figure 50.7. Charcot-Bouchard aneurysm evidenced from tissue obtained after putaminal hematoma evacuation.
P = vessel of microaneurysm origin.
The most common sites for arteriolar damage are the bifurcation of penetrating vessels such as the lenticulostriatal, the thalamic perforating arteries, and the brainstem penetrating arterioles. Previous changes match the common topography of ICH in the basal ganglia (35-45%), cerebral lobes (20-25%), thalamus (10-15%), cerebellum (5-10%) and pons (5%) (Figure 50.8).
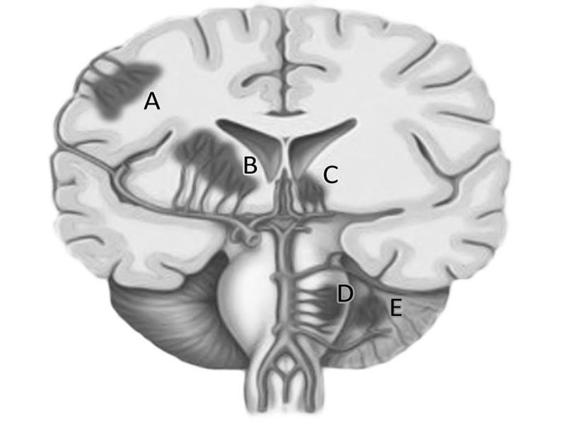
Figure 50.8. Location of hypertensive ICH hemorrhage. Reproduced with permission of Qureshi [12].
A = lobar; B = basal ganglia; C = thalamus; D = protuberance; E = cerebellum.
Other ICH-associated conditions are CVM rupture, hematological dyscrasias, anticoagulants or antiplatelet use, intratumoral bleeding, cerebral venous thrombosis, alcohol intake, legal or illegal use of sympathomimetic drugs, primary or secondary vasculitis of the central nervous system (CNS) and endocarditis [1,12]. When the cause cannot be defined due to inconsistent or insufficient findings, ICH is considered as undetermined. When all diagnostic procedures are negative, the etiology is considered cryptogenic or idiopathic. Uncommon causes of ICH are listed in Table 50.2 [1,12].
|
Table 50.2. Uncommon causes of ICH.
In a daily practice, ICH location, patient age plus other associated conditions may suggest a possible etiology. For example, lobar ICH in a young patient is strongly suggestive of a CVM rupture; whereas lobar ICH in a non-hypertensive aged subject leads to consider amyloid angiopathy [1,12,30,31].
In a fronto-orbital ICH, it is mandatory to discard aneurysmal rupture, mainly in the presence of interhemispheric or basal subarachnoid hemorrhage. A hematoma with important perilesional edema will orientate toward discarding brain tumor as a cause of ICH. Basal ganglia hemorrhage in a young pregnant woman with pre-eclampsia is highly suggestive of a hypertensive cause or puerperal vasculopathy; in such patients a lobar ICH location must orientate toward considering cerebral venous thrombosis (Table 50.3).
Age | Location | Etiology |
Young age | Lobar | CVM |
Age >55 not hypertensive | Lobar | Amiloid angiopathy |
Adult patient | Ganglionar | Hypertension (78-88%) |
Young age | Ganglionar | Hypertension (11%) |
Adult hypertensive | Lobar | Hypertension (20-30%) |
Young age | Cerebellum | CVM |
Adult hypertensive | Cerebellum | Hypertension |
Young/toxemia | Ganglionar | Hypertension |
Young/puerperium | Lobar | Cerebral venous thrombosis |
Elderly subject | Any location | Tumor |
Table 50.3. Etiology by age and location of ICH plus perilesional edema.
50.8 Pathophysiology of ICH
ICH is a dynamic and complex process of simultaneous or consecutively interrelated events which can be grouped into the following phases:
a. Hematoma formation. This occurs whitin the first 60 minutes. Irruption of blood into the brain destroys the parenchymal tissue, alters intracerebral homeostasis, triggers an increase in local pressure with mass effect, leading to displacement of vectors and distortion of intracranial structures. In addition, the hemorrhage by itself is the source of three other deleterious events: cellular death by necrosis and apoptosis, inflammation and vasogenic edema [32-42].
b. Hematoma expansion. In 38% of patients, hematoma continues to grow after the initial event in the first 3 hours, which is associated with neurological deterioration and poor prognosis. In two thirds of such cases, hemorrhage expansion is already evident in the first hour (Figure 50.9) [43].
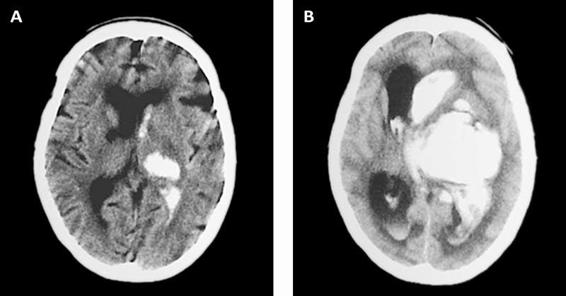
Figure 50.9. Hematoma expansion observed in the first hours.
Hematoma expansion occurs in the absence of coagulopathies. Although the mechanisms by which this occurs are not yet fully understood, it seems to be due to continuous bleeding from the initial site of the ICH or by small “satellite” hemorrhages in the periphery of the clot [44,45].
Other risk factors or conditions contributing to hematoma growth are issues of meticulous analyses, both in clinical and molecular scenarios, and they represent an opportunity area for therapeutic interventions. Alcohol abuse, hyperglycemia, reduced prothrombin activity, hypofibrinogenemia and liver diseases have all been associated with this phenomenon [43,46]. The “spot sign” as evidenced by CTA, early hematoma density and shape are predictors of hematoma expansion that require further studies to confirm its precise value [20,28].
c. Edema. It appears after 24 hours around the hematoma and reaches its major expression at the fourth day, before slowly decreasing [32,33,35-37,39-42,45]. Severe edema develops mainly in the white matter, is primarily vasogenic, and results from increased blood-brain barrier permeability due to its destruction or dysfunction and the action of neurotoxic substances like thrombin or metalloproteinases released from the extracellular matrix.
For many years, “peri-hematoma’’ edema was considered as an “ischemic penumbra’’ area, similar to that present in cerebral infarcts; nevertheless, SPECT (single-photon emission computed tomography), PET (positron emission tomography), diffusion-perfusion MRI techniques and microdialysis have all reliably concluded that this zone corresponds to a regional reduction in brain blood flow resulting from the concomitant decrease in metabolic demand or the presence of mitochondrial dysfunction [35,36,41,47].
50.9 Medical Treatment of ICH
The treatment for all types of ICH is medical, possibly surgical, and will preferably be carried out in a neurointensive care unit [48]. For surgical approaches, patient age and co-morbidities are important issues, as are the neurological status at emergency room arrival, hematoma location and volume, displacement of midline structures, ventricular irruption, hydrocephalus, etiology and patient or family end-of-life preferences [1,12,23]. In spite of these variables, some of which easily measurable, ICH treatment remains uncertain [49,50].
50.9.1 Initial Treatment of ICH
The objective is to reduce intracranial pressure and to avoid neurological and systemic complications [23,27]. Management begins with general measures (ABC approach), airway control for optimal ventilation and oxygenation for hemodynamic stabilization. A brief neurological examination must include assessment with the Glasgow Coma Scale (GCS) or the National Institutes of Health Stroke Scale (NIHSS), as well as the evaluation of pupillar form, size and reactivity, together with the respiratory pattern. The decision whether to institute endotracheal intubation is based on clinical judgment, since its delay aggravates the pre-existing neurological damage and increases morbidity and mortality as a result of the secondary insult from hypoxemia, hypercapnia or aspiration pneumonia [11,39,43,44,51-59].
Hypotension needs to be immediately corrected with iso- or hypertonic fluids, colloids and amines such as phenylephrine, dopamine or norepinephrine to maintain adequate cerebral perfusion pressure. The following laboratory parameters must be obtained: hemogram, glycemia, urea, serum creatinine, electrolytes, coagulogram (including platelet count, prothrombin time, partial thromboplastin time and INR), serum cardiac enzymes, arterial gasometry and toxicological profile (in cases of suspected drug abuse). The electrocardiogram and chest x-ray are useful to evaluate cardiovascular function in patients with or without a history of arterial hypertension in the preoperative work-up, and also to detect early non-neurological complications (arrhythmias, pneumonia) [23]. Under no condition should an unstable patient be transferred for complementary studies.
Once obtained, cranial CT findings must be analyzed jointly by the neurosurgery team to decide the initial management according to the flowchart shown in Figure 50.10.
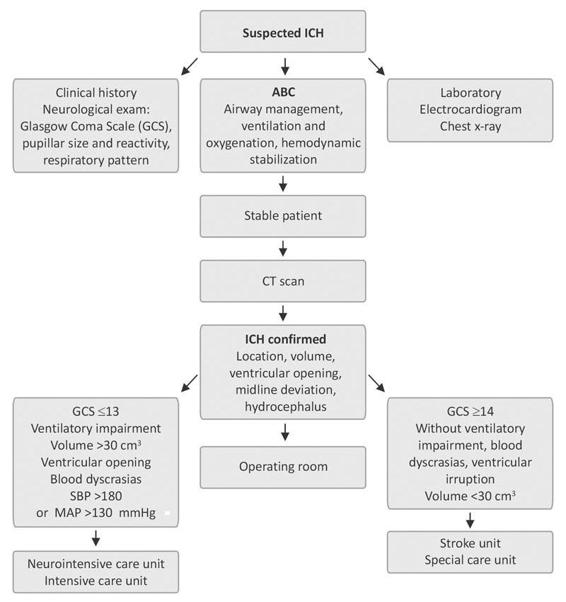
Figure 50.10. Initial management of intracerebral hemorrhage.
SBP= systolic blood pressure; MAP= mean arterial pressure.
50.9.2 Medical Treatment of ICH in Critical Care Units
General Measures
The general measures of care for ICH patients are similar to those for other acute cerebral insults [23,51,58]. It is recommended to establish a central venous route, high calibre peripheral pathways and an arterial line. The advantages of this approach are that it allows for the precise monitoring of volemia and average arterial tension for easy serial extraction of blood samples without the need for extra procedures on the patient; it also helps when volume expanders are required, colloids, mannitol, plasma or total blood without interfering with the simultaneous administration of sedatives, analgesics, antihypertensives or other drugs.
With regard to the use of intravenous solutions, isotonic sodium chloride 0.9% (physiological solution) or slightly hypertonic solution (3.5%) is recommended, depending on various different factors, including natremia, cerebral salt-wasting syndrome, inappropriate antidiuretic hormone secretion syndrome, endocranial hypertension, volemia, mass effect on CT (midline deviation, cistern effacement), etc. Under no circumstances (except in hypoglycemia) should hypotonic solutions such as dextrose or Ringer´s lactate be given because they worsen the cerebral edema, promote the synthesis of neurotoxic neurotransmitters such as glutamate, produce local tissue acidosis (a powerful stimulator of cerebral vasodilatation), and increase cerebral blood volume with elevation of intracranial pressure [23,51,58]. The “6 N” or “six normalities’’ serves as a mnemonic rule (Figure 50.11).
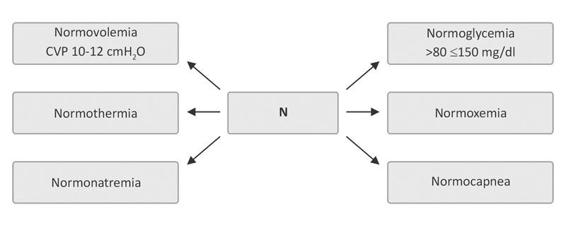
Figure 50.11. The “6 N”, the mnemonic rule to keep in mind when taking care of ICH patients.
CVP= central venous pressure.
Fever must be aggressively treated because of its deleterious effects on neurons and the blood-brain barrier: stimulation of the production of free radicals and elevation of excitatory amino acid levels. Fever also increases the cerebral metabolic demands of oxygen, with repercussions on intracranial pressure, in addition to decreasing the seizures threshold [60].
Hyperthermia has been associated with poor prognosis [61]. Studies by Godoy et al. have demonstrated a close relationship between the systemic inflammatory response syndrome (SRIS) as a predictor of worse prognosis and mortality in ICH, with fever being a component of the syndrome [62]. The authors recommend its management by physical means, thermal blankets, and the administration of acetaminophen in doses of up to 2 g daily when rectal temperature exceeds 37.5°C. This practice is in line with recent results of a multicenter study on acetaminophen in stroke [63].
Assisted mechanical ventilation is indicated in patients with one or more of the following criteria: GCS ≤8; neurological deterioration (defined as worsening of 2 or more GCS points); endocranial hypertension; respiratory insufficiency; abnormal ventilatory pattern; inability to obtain normoxemia and normocapnea, in spite of additional airway support; and immediate postoperative measures.
If a tracheotomy is created, independently of the time at which it is indicated, it should be done by the percutaneous route. On the other hand, due to the hypercatabolic state and to the difficulty in swallowing in these patients, feeding must be initiated early, within the first 48 hours and preferably by the enteral route with a nasojejunal tube. Gastrokinetics (e.g., methoclopramide or cinitapride) are recommended for concomitant gastroparesis caused by the primary brain damage, the release of cytokines and other mediators, as well as the routine use of opioids or phenytoin, [23,51,58].
Additionally, and despite recent controversies, the head should be maintained 30 degrees from the horizontal plane in a “neutral” position, i.e., without anteroposterior or lateral flexion-extension, to improve the volume-pressure ratio of the cranial cavity, venous drainage, and the redistribution of cerebrospinal fluid; this measure also reduces the probability of mycroaspiration and mechanical ventilation-induced pneumonia.
Other equally important measures [58] include the use of artificial eye drops and frequent eyewashes, frequent oral hygiene with clorhexidine or other similarly effective mouthwashes. To prevent the development of pressure ulcers, the patient’s position should be regularly changed – an air or water mattress is not a substitute for this basic care practice. Stress ulcer prophylaxis can be achieved with H2 blockers, proton pump inhibitors or contact antacids such as sucralfate. Deep venous thrombosis prophylaxis can be achieved by using elastic bandages or pneumatic sleeves with sequential compression. In patients at high risk for thrombembolism such as those with obesity, prolonged immobilization, or hip fracture among others, subcutaneous heparin administration or low-molecular-weight heparinoids are recommended after first 24 hours of ICH.
Specific Measures
Normoglycemia
The strict control of glycemia levels is associated with multiple benefits, including the prevention of osmotic diuresis, maintenance of neutrophil and macrophage function, reduced free radicals production, increased nitric oxidase production and improved erythropoiesis [44,54,55,58]. Nevertheless, studies suggesting an aggressive control of hyperglycemia have not been conducted in ICH. Godoy and his group recommend strict glycemia monitoring and treatment with current insulin therapy if glycemia >150 mg/dl levels [64-66].
Anticonvulsants
Seizure frequency in ICH varies from 5 to 28% depending on the diagnosis method, being the highest when continuous electroencephalography is conducted [23,43,46,52]. Although anticonvulsive prophylaxis in ICH is controversial, the use of anticonvulsants is recommended in the following conditions: seizures as the presenting symptom of ICH, history of epilepsy or seizures with or without anticonvulsants use, lobar ICH with cortical extension and during the postoperative period [54-59,66-69]. Anticonvulsants are indicated in the first month and should be increased later. If seizures recur, the condition must be treated as an epileptic form.
Hemostatic Therapy
Recombinant activated factor VII (rFVIIa or NovoSeven®) is a powerful hemostatic activator used in the treatment of hemophilia [43]. rFVIIa acts primarily in endothelial damaged sites, promoting complexes formation with exposed tissue factors [43,46]. These properties were initially promising for ICH patients, especially in the expansion phase of hematoma in the first hours of evolution [46,66,69-71]. Nevertheless, the high expectations generated by a rFVIIa phase II study were not supported by the results of a recent phase III study [71,72]. Although the expansion of hematoma was reduced in the patient group treated with rFVIIa, no functional benefit was observed at 90 days. On the other hand, the high frequency of venous and arterial thromboembolic adverse events observed in the rFVIIa arm, in both the phase II and III studies, are an important issue for its present recommendation [71,72].
Management of Coagulopathies
Patients anticoagulated with coumarin or warfarin have a 5 to 10 times higher risk of a life-threatening ICH, making the reversion of their effects urgent, with both intravenous vitamin K at a daily dose 2 mg; fresh frozen plasma of up to 15 ml/kg body weight (4 or 5 units), or the concentrated infusion of coagulation factors II, VII, IX and X [11,54,58,66,67,71]. Management should never be delayed while waiting for coagulation tests, the combined use of all available therapeutic tools being frequent in daily practice.
Heparin is inactivated by its antagonist, the sulfate of protamine at 1 mg for every 100 units of heparin. Patients with thrombocytopenia or platelet dysfunction can be treated with platelet concentrate infusion or with a unique dose of 0.3 μg/kg of desmopresine acetate.
Blood Pressure Management
Few aspects in the acute phase of ICH management have generated as much controversy as the handling of blood pressure. Some authors consider that high values are associated with hematoma expansion, greater peri-hematoma edema, and an increased risk of recurrence. Others have argued that its decrease is associated with ischemia due to reduced brain perfusion [11,73,74]. Although the American Heart Association (AHA) guidelines suggest the blood pressure values for which treatment must be initiated, their recommendations lack evidence (level V, degree C), since they were reviewed in patients with ischemic stroke and did not undergo substantial modifications [23,51].
Recent works propose a systolic blood pressure <160 mmHg which, besides being safe, reduces the probability of rebleeding [73-75]. Based on class IV evidence, the 2005 European Stroke Initiative (EUSI) recommends the following [58]: the blood pressure reduction as a routine is not recommended; in chronic hypertensive patients, blood pressure must be treated when systolic and diastolic values exceed 180/105 mmHg, respectively, with a mean arterial pressure (MAP) of 125 mmHg as a target; in non-hypertensive patients, blood pressure values >160/95 mmHg must be treated with the objective to reach to reach a blood pressure of 150/90 mmHg or a MAP of 110 mmHg. Reductions >20% of basal values must be avoided.
Both guidelines agree on early and aggressive treatment of blood pressure when one or more of the following conditions exist: acute pulmonary edema; aorta dissection; acute myocardial infarction; hypertensive encephalopathy; and acute renal failure [51,58].
With regard to antihypertensive drugs use, the literature recommends labetalol as a first-line election, followed by esmolol or nicardipine. The use of nitroprusside sodium and nitroglycerin must be avoided since they have a remarkable vasodilator effect on the cerebral vasculature [51,58]. Clonidine, a low-cost drug accessible and available in almost all intensive care units, is safe in spite of a weak evidence of use [54]; a load drug administration must be avoided, since it can stimulate peripheral receptors with a rebound effect. Clodinine is a presynaptic alpha-agonist that, in addition to its antihypertensive effects, also contributes to mitigate the sympathetic hyperactivity observed in these patients, besides having the advantage of an antiemetic, analgesic, and mild sedative effects. The conventional dose is 0.2 to 0.5 μg/kg/min.
The ATACH (Antihypertensive Treatment of Cerebral Acute Hemorrhage) study of safety and feasibility demonstrated that nicardipine by the intravenous route is viable in the strict control blood pressure [75]. However, we must await the results from the larger scale study (ATACH-2) to confirm these findings and to know its impact on mortality and disability in the short, medium and long term.
A similar pilot study, titled INTERACT (Cerebral Intensive Blood Pressure Reduction in Acute Hemorrhage Trial), showed the safety in the intensive management of systolic blood pressure values when they are reduced up to 140 mmHg [76]. The clinical impact is now analyzed under the scope of another study (INTERACT-2). The medical treatment of ICH in the neurointensive care unit is summarized in Figure 50.12.
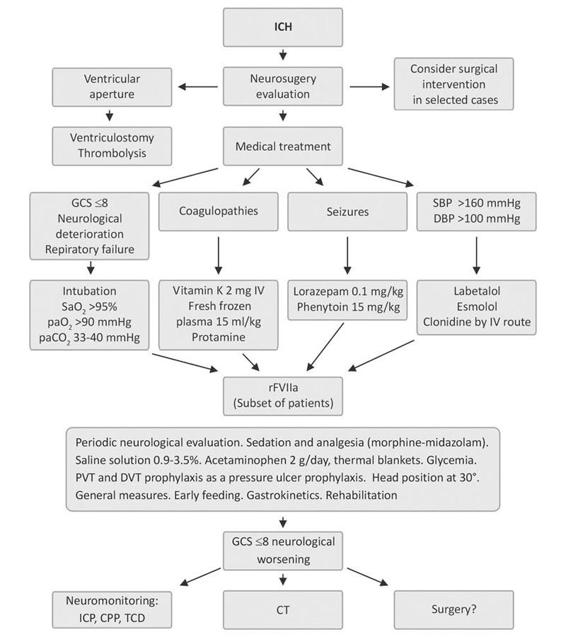
Figure 50.12. Medical treatment of ICH in the neurointensive care unit.
CPP = cerebral perfusion pressure; CT= computed tomography; DBP = diastolic blood pressure; DVT = deep venous thrombosis; GCS = Glasgow Coma Scale ICP = intracranial pressure; PVT = pulmonary venous thrombosis; SBP = systolic blood pressure; TCD = transcranial doppler.
Independently of surgical hematoma drainage or not, sequential intracranial hypertension management is indicated (Figure 50.13). It begins with general measures (sedation, analgesia, hyperthermia and seizures control while avoiding hypoxemia, and hypercapnia). If intracranial hypertension persists, ventricular drainage up to 20 ml/h is recommended, in addition to the use of 7.5% hypertonic saline solution as osmotherapy, together with mild hyperventilation, maintaining CO2 levels between 30 and 35 mmHg. The use of muscle relaxants is not mandatory unless strictly needed. A new CT can be obtained at any time as deemed necessary by the neurosurgery staff. All these therapeutic approaches are considered “first level measures” and a poor response is associated with poor prognosis [23,52,54,56,77].
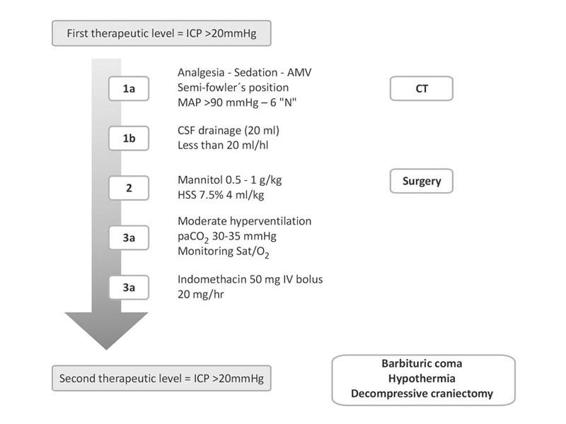
Figure 50.13. Flowchart for intracranial hypertension management in the neurointensive care unit.
AMV = assisted mechanical ventilation; CSF = cerebrospinal fluid; CT = computed tomography; HSS= hypertonic saline solution; ICP= intracranial pressure; MAP = mean arterial pressure.
50.10 Surgical Treatment of ICH
The surgical treatment of the ICH is highly controversial, especially for supratentorial hematomas [34,49,77]. The hypothetical benefits of surgical drainage are that it decreases the hematoma mass effect, avoiding the release of neurotoxic products and preventing prolonged tissue-blot interaction, besides improving regional blood flow and peri-hematoma brain metabolism [78]. Nevertheless, these theoretical advantages must be weighed against the inevitable damage of normal parenchyma to access the hemorrhage [40,79-81]. Most neurosurgeons agree on the need to drain lobar or cerebellar hematomas in patients with clinical deterioration. What remains uncertain is the appropriate surgical approach to a ganglionic ICH [40,79,80].
The AHA guidelines [23,51] recommend surgical treatment in the following conditions: cerebellar hematomas >3 cm in diameter in patients who clinically deteriorate, have hydrocephalus, secondary brainstem or fourth ventricle compression (evidence C); secondary CVM hematomas; medium to larger volume lobar hematomas in young patients with neurological worsening.
The lack of studies with suitable methodology had lead mainly to low-evidence studies, some of which date from the pre-CT era or were derived from a few patients series [82-85]. Recent studies such as the STICH (International Surgical Trial in Intracerebral Hemorrhage) could not satisfy this question [50]. In this multicentre, international study, 1033 patients with supratentorial ICH within the first 72 hours were recruited and randomized to medical versus surgical treatment. Based on the uncertainty or doubt principle with respect to the utility of surgery, all those patients in which neurosurgeons were convinced of the benefits of surgery were operated and not included in the study. The average time from ICH onset to surgery was 30 hours. The results showed no difference in mortality or incapacity at 3 months, which is why the results of this study do not change the present practice [50].
A small subgroup of STICH patients with a better evolution was, however, identified: those with a GCS score between 9 and 12, with more superficial hematomas located to ≤1 cm of the brain cortex. An ongoing collaborative study in lobar ICH (STICH-2) is now comparing medical versus surgical treatment.
50.10.1 Ventricular Extension of ICH
Ventricular extension of ICH occurs in about 40% of patients, mainly when located deep in the thalamus, caudate, putamen or cerebellum [43,44,56,86-91]. Intraventricular hemorrhage carries a poor prognosis, nevertheless, it is the volume and not just its presence that determines the predictive value, being invariably fatal when >20 ml [67,86,88-92]. Its deleterious effects are associated with the development of hydrocephalus, intracranial hypertension, and ischemia of the cortical structures [86,87,91,93]. External ventricular drainage is a therapeutic option; however, its beneficial role remains uncertain.
The permeability of ventriculostomy is difficult to maintain due to frequent obstruction by clots, which is why different studies have used fibrinolytics like urokinase, streptokinase and rtPA [86,88]. Various studies in small series have shown good results related to a minor use of permanent peritoneal derivations and a reduction in mortality with acceptable functional prognosis; nevertheless, despite the high risk of complications such as infections or hemorrhages, prospective multicentre trials, have validated the effectiveness of this approach.
50.10.2 Hydrocephalus
Hydrocephalus develops due to the external compression of the ventricular system by the mass effect of hematomas, mainly those located in the thalamus or putamen which deviate the midline and compress the foramen of Monro, and also by small hemorrhages near of ventricular system as occur in the cerebellar vermis. The second mechanism is by clots obstructing the normal circulation of cerebrospinal fluid [93,94].
Independently of its cause, hydrocephalus leads to intracranial hypertension, cerebral ischemia and neurological deterioration. In addition, it is an independent predictive factor of mortality and inauspicious evolution, mainly when associated with supratentorial hemorrhages. Hydrocephalus is frequently treated with ventriculostomy; nevertheless, the benefit of this therapeutic modality has not been established. New alternative approaches utilizing minimally invasive procedures such as simple drainage by stereotaxia and thrombolysis with hematoma aspiration are technically promising but lack sufficient evidence for their generalized use.
Reference | n | Scale components | Mortality within 30 days (%) |
Hemphill JC et al, 2001 | 152 | 1. Glasgow Coma Scale at entrance 2. ICH volume 3. Irruption to the ventricular system 4. Infratentorial lCH 5. Age | 45 |
Cheung RTF et al, 2003* | 142 | 1. NIHSS at entrance 2. ICH volume 3. Irruption to the ventricular system 4. Infratentorial ICH 5. Age** | 22 |
Shaya M et al, 2004 | 50 | 1. ICH volume 2. Hydrocephalus 3. Neurological focal deficit | 18 |
Weimar C et al, 2006* | 340 | 1. Age 2. NIHSS at entrance 3. Consciousness level on NIHSS | 37 |
Godoy DA et al, 2006* | 153 | 1. Glasgow Coma Scale at entrance 2. ICH volume 3. Irruption to the ventricular system 4. Intraventricular ICH (Graeb’s scale) 5. Age | 35 |
Ruiz-Sandoval JL et al, 2007* | 378 | 1. Glasgow Coma Scale at entrance 2. ICH volume 3. Irruption to the ventricular system 4. Infratentorial lCH 5. Age | 47 |
Table 50.4. Prognostic scales for mortality in ICH.
* Scales designed in addition for predicting good functional outcome;
** Other variables also were proved.
Surgical treatment of secondary ICH due to rupture of arteriovenous malformations (AVMs), cavernous or venous angiomas is not usually urgent. A relatively low mortality in the acute phase of ICH due to AVMs (8.9-19.2%), associated with the reduced risk of immediate recurrence (3-17% for the first year and 2-6% thereafter), as well as the absence of vasospasm, makes time a non-critical condition for their treatment [95]. Mortality associated with ICH due to cavernous angiomas is similar that from AVMs; nevertheless, the risk of recurrence of bleeding is less (0.25-0.7% per year for non-familiar supratentorial cavernous angiomas and up to 6.5% per year for familiar cases) with a risk of recurrence of bleeding of 1.3% depending on the number of lesions per year [96]. The better prognosis relies on closer follow-up, waiting for clot reabsorption, with control studies (MRI) and careful planning of an eventual surgery in candidate cases.
50.11 Mortality and Prognosis in ICH
Acute mortality in ICH varies from 20 to 70%, being many factors its determinants [1,9-11]. A wide range of clinical, molecular and tomographic markers have been identified as predictors of mortality. The GCS score and the volume of the hematoma are variables with the highest weight and consistency in different cohorts. Recently, and by coincidence with this so called “forgotten pathology”, there has been renewed interest in the proposal of several systems for predicting mortality in-hospital and at 30, 90 and 180 days after ICH. Table 50.4 shows the prognostic scales for 30-day mortality [91,92,97-99].
Some of these scales also were developed for predicting a good evolution and none of them have been validated in cohorts independently. The scale proposed by Hemphill [92] is one of the most accepted at the moment, since is simple to apply and is reproducible and highly predictive (Table 50.5). The scale has been validated and modified by others in different scenarios, including the proposals by the authors of this chapter [97-99].
The predictive good prognosis at 3 months after ICH is similar to the findings at in-hospital or at 30 days evolution. A recent scale, denominated the FUNC score (Functional Prediction of Outcome in Patients with Primary Intracerebral Hemorrhage) demonstrated that at 3 months, poor or good prognosis is associated with age, GCS at emergency room arrival, hemorrhage location and volume, in addition to previous cognitive deterioration (a good prognosis was based on a GCS >4) [100].
Points | ||
Glasgow score | 3-4 | 2 |
5-12 | 1 | |
13-15 | 0 | |
Volume (cm3) | ≥30 | 1 |
<30 | 0 | |
Ventricular opening | Yes | 1 |
No | 0 | |
Infratentorial | Yes | 1 |
No | 0 | |
Age (years) | ≥80 | 1 |
<80 | 0 | |
Total | 0-6 | |
Death probability at 1 month (ICH score) | ||
Points | % | |
0 | – | |
1 | 13 | |
2 | 26 | |
3 | 72 | |
4 | 97 | |
5 | 100 | |
6 | 0 | |
Table 50.5. Hemphill’s prognostic scale for ICH (ICH score).
50.12 Conclusion
ICH is the most catastrophic of all forms of CVD. It still is “the great forgotten one” in spite of recent interesting studies. It seems to be more frequent in Latin America than in other countries, calling for the need to design incidence studies that could confirm it. Risk factors, clinical presentation and radiological findings are uniform in all scenarios. The best medical and surgical management remains uncertain. There is an urgent need for the initiation, termination and validation of all local or multicentre studies that could confirm or reject currently proposed therapeutic options such as neuroprotection, hemostatics use, blood pressure control, and the best surgical instrumentation with consequent safe and effective access.
References
1. Kase CS, Caplan LR (eds). Intracerebral hemorrhage. Boston: Butterworth-Heinemann; 1994
2. SHEP Cooperative Research Group. Prevention of various stroke types by treatment of isolated systemic hypertension. International Stroke Society’s Second World Congress of Stroke 1992, Washington DC. JAMA 1991; 265: 3255-64
3. Broderick JP, Brott T, Tomsick T, et al. The risk of subarachnoid and intracerebral hemorrhage in black as compared with whites. N Engl J Med 1992; 326: 733-6
4. American Heart Association Medical/Scientific Statement, Special report. Cardiovascular diseases and stroke in African Americans and other racial minorities in the United States. Circulation 1991; 83: 1462-80
5. Bruno A, Carter S, Qualls C, et al. Incidence of spontaneous intracerebral hemorrhage among hispanics and non-hispanic whites in New Mexico. Neurology 1996; 47: 405-8
6. Del Brutto OH, Mosquera A, Sánchez X, et al. Stroke subtypes among hispanics living in Guayaquil, Ecuador. Results from the Luis Vernaza Hospital Stroke Registry. Stroke 1993; 24: 1833-6
7. Barinagarrementería F, Ruiz-Sandoval JL, Arauz A, et al. A hospital stroke register in Mexico City: analysis of 2045 patients. Neurology 1999; 52 (Suppl. 2): A442. Abstract
8. Saposnik G, Caplan LR, Gonzalez LA, et al. Differences in stroke subtypes among natives and caucasians in Boston and Buenos Aires. Stroke 2000; 31: 2385-9
9. Ruiz-Sandoval JL, Ortega-Alvarez L, García-Navarro V, et al. Hemorragia intracerebral en un hospital de referencia de la región centro-occidente de México. Rev Neurol 2005; 40; 656-60
10. Lavados PM, Sacks C, Prina L, et al. Incidence, 30-day case-fatality rate, and prognosis of stroke in Iquique, Chile: a 2-year community-based prospective study (PISCIS project). Lancet 2005; 365: 2206-15
11. Badjatia N, Rosand J. Intracerebral hemorrhage. Neurologist 2005; 11: 311-24
12. Qureshi AI, Tuhrim S, Broderick JP, et al. Spontaneous intracerebral hemorrhage. N Engl J Med 2001; 344: 1450-60
13. Sacco RL, Mayer SA. Epidemiology of intracerebral hemorrhage. In: Feldman E (ed.). Intracerebral Hemorrhage. Armonk, NY: Futura, 1994; pp. 3-26
14. Abbott RD, Yin Y, Reed DM, et al. Risk of stroke in male cigarette smokers. N Engl J Med 1986; 315: 717-20
15. Iso H, Jacobs DR Jr, Wentworth D, et al. Serum cholesterol levels and six-year mortality from stroke in 350,977 men screened for Multiple Risk Factors Intervention Trial. N Engl J Med 1989; 320: 904-10
16. Culebras A, Kase CS, Masdeu JC, et al. Practice guidelines for the use of imaging in transient ischemic attacks and acute stroke. A report of the Stroke Council, American Heart Association. Stroke 1997; 28: 1480-97
17. Graeb DA, Robertson WD, Lapointe JS, et al. Computed tomographic diagnosis of intraventricular hemorrhage. Etiology and prognosis. Radiology 1982; 143: 91-6
18. Kothari RU, Brott T, Broderick JP. The ABCs of measuring intracerebral hemorrhage volumes. Stroke 1996; 27: 1304-5
19. Broderick JP, Brott TG, Duldner JE, et al. Volume of intracerebral hemorrhage. A powerful and easy-to-use predictor of 30-day mortality. Stroke 1993; 24: 987-93
20. Barras CD, Tress BM, Christensen S, et al; Recombinant Activated Factor VII Intracerebral Hemorrhage Trial Investigators. Density and shape as CT predictors of intracerebral hemorrhage growth. Stroke 2009; 40: 1325-31
21. Zilkha A. Intraparenchymal fluid-blood level: a CT sign of recent intracerebral hemorrhage. J Comput Assist Tomogr 1983; 7: 301-5
22. Wada R, Aviv RI, Fox AJ, et al. CT angiography “spot sign” predicts haematoma expansion in acute intracerebral hemorrhage. Stroke 2007; 38: 1257-62
23. Broderick JP, Adams HP, Barsan W, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: A statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Association. Stroke 1999; 30: 905-15
24. Kucharczyk W, Lemme-Pleghos L, Uske A, et al. Intracranial vascular malformations: MR and CT imaging. Radiology 1985; 156: 383-9
25. Atlas SW, Grossman RI, Gomori JM, et al. Hemorrhagic intracranial malignant neoplasms: spin-echo MR imaging. Radiology 1987; 164: 71-7
26. Rigamonti D, Drayer BP, Jonson PC, et al. The MRI appearance of cavernous malformations (angiomas). J Neurosurg 1987; 67: 518-24
27. Ruiz José Luis, Colorado H, Loy MC, et al. Diagnóstico y tratamiento de la hemorragia intracerebral. Rev Invest Clin 2002; 54: 275-80
28. Vernooij MW, van der Lugt A, Ikram MA, et al. Prevalence and risk factors of cerebral microbleeds. Neurology 2008; 70: 1208-14
29. Knudsen KA, Rosand J, Karluk D, et al. Clinical diagnosis of cerebral amyloid angiopathy: Validation of the Boston criteria. Neurology 2001; 56: 537-9
30. Schütz H, Bödeker RH, Krack P, et al. Age-related spontaneous intracerebral hematoma in a German community. Stroke 1990; 21: 1412-8
31. Ruiz-Sandoval JL, Cantu C, Barinagarrementeria F. Intracerebral hemorrhage in young people: analysis of risk factors, location, causes and prognosis. Stroke 1999; 30: 537-
32. Carhuapoma JR, Barker PB, Hanley DF, et al. Human brain hemorrhage: quantification of perihematoma edema by use of diffusion-weighted MR imaging. Am J Neuroradiol 2002; 23: 1322-6
33. Gebel JM, Jauch EC, Brott TG, et al. Natural history of perihematomal edema in patients with hyperacute spontaneous intracerebral hemorrhage. Stroke 2002; 33: 2631-5
34. Godoy DA, Piñero G. Respuesta inflamatoria en la hemorragia intracerebral espontanea. Rev Neurol 2005; 40: 492-7
35. Powers WJ, Zazulia AR, Videen TO, et al. Autoregulation of cerebral blood flow surrounding acute (6 to 22 hours) intracerebral hemorrhage. Neurology 2001; 57: 18-24
36. Qureshi AI, Wilson DA, Hanley DF, et al. No Evidence for an ischemic penumbra in massive experimental intracerebral hemorrhage. Neurology 1999; 52: 266-72
37. Schellinger PD, Fiebach JB, Hoffman K, et al. MRI in intracerebral hemorrhage. Is there a perihemorrhagic penumbra? Stroke 2003; 34: 1674-80
38. Wang J, Doré S. Inflammation after intracerebral hemorrhage. J Cereb Blood Flow Metab 2007; 27: 894-908
39. Xi G, Fewel ME, Hua Y, et al. Intracerebral hemorrhage. Pathophysiology and therapy. Neurocritical Care 2004; 1: 5-18
40. Xi G, Keep RF, Hoff JT. Mechanisms of brain injury after intracerebral hemorrhage. Lancet Neurol 2006; 5: 53-63
41. Zazulia AR, Diringer MN, Vidden TO, et al. Hypoperfusion without ischemia surrounding acute intracerebral hemorrhage. J Cereb Blood Flow Metab 2001; 21: 804-10
42. Zazulia AR, Diringer MN, Derdeyn CP, et al. Progression of mass effect after intracerebral hemorrhage. Stroke 1999; 30: 1167-73
43. Mayer SA, Rincon F. Treatment of intracerebral hemorrhage. Lancet Neurol 2005; 4: 662-72
44. Hsieh PC, Awad IA, Getch CC, et al. Current updates in perioperative management of intracerebral hemorrhage. Neurol Clin 2006; 24: 745-64
45. NINDS ICH Workshop Participants. Report from a National Institute of Neurological Disorders AND Stroke Workshop. Priorities for Clinical Research in Intracerebral Hemorrhage. Stroke 2005; 36: 23-41
46. Mayer SA, Brun NC, Begtrup K, et al. Recombinant Activated Factor VII Intracerebral Hemorrhage Trial Investigators. Recombinant activated factor VII for acute intracerebral hemorrhage. N Eng J Med 2005; 352: 777-85
47. Kim-Han JS, Kopp SJ, Dugan LL, et al. Perihematomal mitochondrial dysfunction after intracerebral hemorrhage. Stroke 2006; 37: 2457-62
48. Diringer MN, Edwards DF. Admisión to a neurologic/neurosurgical intensive care unit is associated with reduced mortality rate after intracerebral hemorrhage. Crit Care Med 2001; 29: 635-40
49. Fernandes HM. Surgery in intracerebral hemorrhage. The uncertainty continues. Stroke 2000; 31: 2511-9
50. Mendelow AD, Gregson BA, Fernández HM, et al; STICH Investigators. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial intracerebral haematomas in the International Surgical Trial in Intracerebral Hemorrhage (STICH): a randomized trial. Lancet 2005; 365: 387-97
51. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Morgenstern LB, Hemphill JC 3rd, Anderson C, et al.; American Heart Association Stroke Council and Council on Cardiovascular Nursing. Stroke 2010; 41: 2108-29
52. Butcher K, Laidlaw J. Current intracerebral hemorrhage management. J Clin Neuroscience 2003; 10: 158-67
53. Fewel ME, Thompson G, Hoff JT. Spontaneous intracerebral hemorrhage: a review. Neurosurg Focus 2003; 15: 1-16
54. Godoy DA, Badolatti A. Hemorragia Intracerebral espontanea. Tratamiento Medico. Manual de Neurointensivismo. Sociedad Argentina de Terapia Intensiva. Editorial Médica Panamericana. In press
55. Grotta J. Management of primary hypertensive hemorrhage of the brain. Curr Treat Options Neurol 2004; 6: 435-42
56. Manno EM, Atkinson JLD, Fulgham JR, et al. Emerging medical and surgical management strategies in the evaluation and treatment of intracerebral hemorrhage. Mayo Clin Proc 2005; 80: 420-33
57. Rincon F, Mayer SA. Novel therapies for intracerebral hemorrhage. Curr Opin Crit Care 2004; 10: 94-100
58. The European Stroke Initiative Writing Committee and the writing committee for the EUSI Executive committee. Recommendations for the management of intracranial hemorrhage-Part I: Spontaneous Intracerebral hemorrhage. Cerebrovas Dis 2006; 22: 294-316
59. Towfighi A, Greenberg SM, Rosand J. Treatment and prevention of primary intracerebral hemorrhage. Seminars Neurology 2005; 25: 445-52
60. Marion DW. Controlled normothermia in neurologic intensive care. Crit Care Med 2004; 32 (suppl 2): S43-S45
61. Schwarz S, Hafner Kaschoff A, Schwab S. Incidence and prognostic significance of fever following intracerebral hemorrhage. Neurology 2000; 54: 354-61
62. Godoy DA, Piñero G. Respuesta inflamatoria en la hemorragia intracerebral espontanea. Rev Neurol 2005; 40: 492-7
63. den Hertog HM, van der Worp HB, van Gemert HM, et al; on behalf of the PAIS investigators. The Paracetamol (Acetaminophen) In Stroke (PAIS) trial: a multicentre, randomised, placebo-controlled, phase III trial. Lancet Neurol 2009; 8: 434-40
64. Garg R, Chaudhuri A, Munschauer F, et al. Hyperglycemia, insulin, and acute ischemic stroke. A mechanistic justification for a trial of insulin infusion therapy. Stroke 2006; 37: 267-73
65. Godoy DA, Piñero GR, Svampa S, et al. Early hyperglycemia and intravenous insulin—The rationale and management of hyperglycemia for spontaneous intracerebral hemorrhage patients: Is Time for Change? Neurocrit Care 2009; 10: 150-3
66. Juvela S, Kase CS. Advances in intracerebral hemorrhage management. Stroke 2006; 37: 301-4
67. Telleria-Diaz A. Tratamiento e indicadores pronosticos del paciente con hemorragia intracerebral espontanea. Rev Neurol 2006; 42: 341-9
68. Vespa PM, O’Phelan K, Shah M, et al. Acute seizures after intracerebral hemorrhage. A factor in progressive midline shift and outcome. Neurology 2003; 60: 1441-6
69. Sung CY, Chu NS. Epileptic seizures in intracerebral hemorrhage. J Neurol Neurosurg Psychiatry 1989; 52: 1273-6
70. You H, Al-Shahi R. Hemostatic drug therapies for acute primary intracerebral hemorrhage. Cochrane Database Syst Rev 2006; 3: CD005951
71. Friedriksson K, Norrving B, Strömblad L-G. Emergency reversal of anticoagulation after intracerebral hemorrhage. Stroke 1992; 23: 972-7
72. Mayer SA, Brun NC, Begtrup K, et al; FAST Trial Investigators. Efficacy and Safety of Recombinant Activated Factor VII for Acute Intracerebral Hemorrhage. N Engl J Med 2008; 358: 2127-37
73. Carhuapoma JR, Ulatowski JA. Blood pressure control after intracerebral hemorrhage: Have we reached the target? Crit Care Med 2006; 34: 2023-4
74. Ohwaki K, Yano E, Nagashima H, et al. Blood pressure management in acute intracerebral hemorrhage. Relationship between elevated blood pressure and hematoma enlargement. Stroke 2004; 35: 1364-7
75. Qureshi AI. Antihypertensive Treatment of Acute Cerebral Hemorrhage (ATACH): rationale and design. Neurocrit Care 2007; 6: 56-66
76. Anderson CS, Huang Y, Wang JG, et al; INTERACT Investigators. Intensive blood pressure reduction in acute cerebral hemorrhage trial (INTERACT): a randomised pilot trial. Lancet Neurol 2008; 7: 391-9
77. Kase CS. Treatment of cerebral hemorrhage. In: Ginssberg M, Bogousslavsky J, ed. Cerebrovascular disease. Pathophysiology, diagnosis and management. Massachusetts: Blackwell Science, 1998; pp. 2022-8
78. Nehls DG, Mendelow AD, Graham DI, et al. Experimental intracerebral hemorrhage: early removal of spontaneous mass lesion improves late outcome. Neurosurgery 1990; 27: 674-82
79. Taneda M, Hayakawa T, Mogami H. Primary cerebellar hemorrhage: quadrigeminal cister obliteration on CT scans as predictor of outcome. J Neurosurg 1987; 67: 545-52
80. Carvi y Nievas MN. Why, when and how spontaneous intracerebral hematomas should be operated. Med Sci Monit 2005; 11: 24-31
81. Donnan GA, Davis SM. Surgery for intracerebral hemorrhage: an evidence-poor zone. Stroke 2003; 34: 1569-70
82. McKissock W, Richardson A, Taylor J. Primary intracerebral hemorrhage: a controlled trial of surgical and conservative treatment in 180 unselected cases. Lancet 1961; 2: 221-6
83. Auer LM, Deinsberger W, Niederkorn K, et al. Endoscopy surgery versus medical treatment for spontaneous intracerebral hematoma: a randomized study. J Neurosurg 1989; 70: 530-5
84. Juvela S, Heskanem O, Poranen A, et al. The treatment of spontaneous intracerebral hemorraghe: a prospective randomized trial of surgical and conservative treatment. J Neurosurg 1989; 70: 755-8
85. Batjer HH, Reisch JS, Allen BC, et al. Failure of surgery to improve outcome in hypertensive putaminal hemorrhage: a prospective randomized study. Arch Neurol 1990; 47: 1103-6
86. Engelhard HH, Andrews CO, Slavin KV, et al. Current management of intraventricular hemorrhage. Surg Neurol 2003; 60: 15-22
87. Mayer SA, Kessler DB, Van Heertum RL, et al. Effect of intraventricular blood on global cortical perfusion in acute intracerebral hemorrhage: a single-photon emission computed tomography study. Ann Neurol 1995; 38: 288. Abstract
88. Naff NJ, Hanley DF, Keyl PM, et al. Intraventricular thrombolysis speeds blood clot resolution: results of a pilot, prospective, randomized, doubled-blind, controlled trial. Neurosurgery 2004; 54: 577-84
89. Tuhrim S, Horowitz DR, Sacher M, et al. Volume of ventricular blood is an important determinant of outcome in supratentorial intracerebral hemorrhage. Crit Care Med 1999; 27: 617-21
90. Young WB, Lee KP, Pessin MS, et al. Prognostic significance of ventricular blood in supratentorial hemorrhage: a volumetric study. Neurology 1990; 40: 616-9
91. Godoy DA, Piñero G, Di Napoli M. Predicting mortality in spontaneous intracerebral hemorrhage. Can modification to original score improve the prediction? Stroke 2006; 37: 1038-44
92. Hemphill JC III, Bonovich DC, Besmertis L, et al. The ICH score: a simple, reliable grading scale for intracerebral hemorrhage. Stroke 2001; 32: 891-7
93. Diringer MN, Edwards DF, Zazulia AR. Hydrocephalus: A previously unrecognized predictor of poor outcome from supratentorial intracerebral hemorrhage. Stroke 1998; 29: 1352-7
94. Phan TG, Koh M, Vierkant RA, et al. Hydrocephalus is a determinant of early mortality in putaminal hemorrhage. Stroke 2000; 31: 2157-62
95. Martin NA. Treatment of arteriovenous malformations: indications, grading, and techniques. J Stroke Cerebrovasc Dis 1997; 6: 272-6
96. Robinson JM, Awad IA, Little JR. Natural history of the cavernous angioma. J Neurosurg 1991; 75: 709-14
97. Cheung RTF, Zou LY. Use of the original, modified, or new intracerebral hemorrhage store to predict mortality and morbidity alter intracerebral hemorrhage. Stroke 2003; 34: 1717-22
98. Weimar C, Benemann J, Diener HC. German Stroke Study Collaboration. Development and validation of the Essen intracerebral hemorrhage score. J Neurol Neurosurg Psychiatry 2006; 77: 601-5
99. Ruiz-Sandoval JL, Chiquete E, Romero-Vargas S, et al. Grading scale for prediction of outcome in primary intracerebral hemorrhages. Stroke 2007; 38: 1641-4
100. Rost NS, Smith EE, Chang Y, et al. Prediction of functional outcome in patients with primary intracerebral hemorrhage: the FUNC score. Stroke 2008; 39: 2304-9
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






