and Héctor H. García3, 4
(1)
School of Medicine, Universidad Espíritu Santo, Santo, Ecuador
(2)
Department of Neurological Sciences, Hospital-Clinica Kennedy, Guayaquil, Ecuador
(3)
Cysticercosis Unit, Instituto Nacional de Ciencias Neurológicas, Lima, Peru
(4)
School of Sciences, Universidad Peruana Cayetano Heredia, Lima, Peru
Abstract
Despite the fact that human taeniasis and cysticercosis have a well-known etiologic agent, diagnosis of these conditions may be a challenge. In the case of taeniasis, most infected individuals are asymptomatic or may develop vague complaints and, as previously described, defining a typical syndrome of cysticercosis is not possible. Such clinical pleomorphism is often complicated by the scarcity of pathognomonic findings or the lack of reliability of many of the available diagnostic tests. Here, we describe the results of the most accepted complementary exams used for the diagnosis of the disease complex taeniasis/cysticercosis.
Despite the fact that human taeniasis and cysticercosis have a well-known etiologic agent, diagnosis of these conditions may be a challenge. In the case of taeniasis, most infected individuals are asymptomatic or may develop vague complaints and, as previously described, defining a typical syndrome of cysticercosis is not possible. Such clinical pleomorphism is often complicated by the scarcity of pathognomonic findings or the lack of reliability of many of the available diagnostic tests. Here, we describe the results of the most accepted complementary exams used for the diagnosis of the disease complex taeniasis/cysticercosis.
8.1 Diagnosis of Taeniasis
Humans carrying the adult Taenia solium in the intestine are the source of cysticercosis infection and should be ruled out in individuals with neurocysticercosis and their household contacts, particularly in young patients or those with massive infestations (Asnis et al. 2009; Del Brutto and Del Brutto 2013a; García and Del Brutto 1999; Gilman et al. 2000). The chances of finding a tapeworm carrier among household contacts of a neurocysticercosis patient are logically smaller in older patients with only calcified lesions, since they may have acquired the infection many years ago and the life span of the adult Taenia solium in tapeworm seems to be below 5 years (Allan et al. 1996a; Huisa et al. 2005).
8.1.1 Microscopy
The traditional parasitological test, i.e., microscopically observation of Taenia eggs, is of poor use for the diagnosis of T. solium taeniasis, even if concentration methods are used. In the best hands, the sensitivity of coproparasitologic microscopy stands below 70 % (García et al. 2003a). Reasons for this include the variable rate of proglottid and egg excretion, that most eggs remain in the proglottids, and the slower growth rate compared to other tapeworms. To partially leverage the poor sensitivity, concentration methods should be used, preferably using sedimentation; larger sample volumes need to be concentrated, and repeated testing (three different samples) is recommended. On the other hand, the specificity of human stool microscopy for taeniasis is very high. Appearance of Taenia egg is quite characteristic, with a thick radiate cover and hooks (embryonic hooks, not cysticercal hooks). However, Taenia solium eggs are extremely similar than those of Taenia saginata; thus a diagnosis of Taenia sp. infection should only be reported. The occurrence of morphological and staining differences between the two tapeworms has recently been suggested, but this is not absolute (Jimenez et al. 2010).
8.1.2 Stool Antigen Detection
Allan et al. (1990, 1993) applied capture ELISA assays to detect Taenia solium antigens in stool samples, as previously reported for Taenia hydatigena and other parasites. This coproantigen-detection ELISA greatly improved the diagnostic sensitivity for human taeniasis. In field surveys, coproantigen detection finds between 1.5 and 2.5 times more taeniasis cases than the classic microscopic stool examination (Allan et al. 1996b; García et al. 2003b). The usual coproantigen ELISA assay uses a polyclonal antibody against the adult tapeworm stage to detect tapeworm antigen in stool supernatant. A 5 % PBS-formaldehyde solution is used as the diluent and fixative to avoid biosafety risks.
The coproantigen-detection assay uses a very low cutoff value calculated from negative samples. Low positive results are frequently nonspecific, but strongly positive samples usually correspond to tapeworm carriers. Samples from Taenia saginata carriers tend to have very low positive results, and only a small proportion of the general population have a consistently positive stool antigen result, likely due to a cross-reaction with rheumatoid factor (Yolken and Stopa 1979). Effective treatment with niclosamide or praziquantel rapidly decreases stool antigen levels, and 2 weeks after treatment almost all cured patients are coproantigen negative; thus, a positive test after 2 weeks or 1 month of treatment marks treatment failure (much before the tapeworm begins to release eggs again) (Bustos et al. 2012).
8.1.3 DNA-Based Assays
The use of PCR assays in the diagnosis of human taeniasis has been described by several groups. These assays can discriminate the species of Taenia and are highly reliable if tapeworm material is available. This is not usually the case, and so far the sensitivity of PCR assays in stool samples is not yet defined (Gonzalez et al. 2000; Mayta et al. 2000; Rodríguez et al. 2012).
8.2 Imaging Diagnosis of Neurocysticercosis
Introduction of modern neuroimaging techniques has greatly increased the diagnostic accuracy for neurocysticercosis by providing objective evidence on the number and topography of lesions, as well as on the activity of the disease, including the degree of the host’s inflammatory reaction against this parasite (Rodriguez-Carbajal and Boleaga-Durán 1982). These imaging methods have replaced previously used radiological procedures such as plain roentgenograms, pneumoencephalograms, ventriculography, conventional myelography, and angiography that currently have only limited or historical importance (Arana and Asenjo 1945; Dorfsman 1963; Trelles and Trelles 1978).
8.2.1 Old Imaging Diagnostic Methods
Small, single, or multiple intracranial calcifications are common on plain X-ray films of the skull in patients with neurocysticercosis (Dixon and Hargreaves 1944; Stepien 1962). These calcifications are typically small and are characterized by an eccentric calcified dot representing the scolex, surrounded by a partially calcified sphere that represents the body of the cystic larva (Santín and Vargas 1966). These characteristics were once considered useful for differentiating calcifications related to cysticercosis from those arising as the result of other infections such as toxoplasmosis or tuberculosis (Rodriguez-Carbajal and Boleaga-Durán 1982). Another finding on plain X-ray films of the skull was the erosion of the sella turcica as a reflection of chronic intracranial hypertension, usually related to hydrocephalus (Cárdenas y Cárdenas 1962). Soft-tissue roentgenograms are still useful for the detection of cysticerci in muscles. Parasites may appear as small punctate or as elliptical, cigar-shaped calcifications (McCormick 1985). These lesions are more frequent in calf and thigh muscles, and in areas where modern neuroimaging diagnostic tests are not readily available, this is a useful radiological sign for the diagnosis of human cysticercosis and provides indirect evidence favoring the diagnosis of neurocysticercosis in patients with neurological complaints (Dumas et al. 1990; Preux et al. 1996).
Pneumoencephalograms and ventriculography were the imaging methods of choice for the diagnosis of intraventricular cysticercosis by identifying filling defects within the ventricular system that corresponded to ventricular cysts. These methods were also of value for the evaluation of the size of the ventricular cavities in patients with suspected hydrocephalus (Bickerstaff et al. 1952; Milenkovic et al. 1982). The main problem was their lack of specificity, since any intraventricular space-occupying lesion may produce similar filling defects.
Myelography was of great diagnostic value in patients with suspected spinal leptomeningeal cysticercosis by showing multiple filling defects in the column of contrast material corresponding to the cysts (Firemark 1978). Using myelography, leptomeningeal cysts may be seen freely mobile within the spinal subarachnoid space and may change their position according to movements of the patient in the exploration table (Kim and Weinberg 1985). However, myelography is not specific in patients with intramedullary cysts of the spinal cord since the method only visualizes partial or complete blockage in the column of contrast material at the level of the lesion, a finding that may also be observed in other diseases of the spinal cord (Akiguchi et al. 1979).
Angiography has been used for the diagnosis of neurocysticercosis since the pioneer description of Moniz et al. (1932). Early reports were most often concerned with the displacement of cerebral arteries caused by hydrocephalus and did not assign a major diagnostic value to this method (Rocca and Monteagudo 1966). More recently, reports dealing with cerebrovascular complications of neurocysticercosis described in detail the spectrum of angiographic changes observed in this parasitic disease, including segmental narrowing or occlusion of small- and middle-size intracranial vessels in patients with cerebral infarctions (Barinagarrementeria and Del Brutto 1989; Levy et al. 1995; Monteiro et al. 1994; Rodriguez-Carbajal et al. 1989) as well as mycotic aneurysms in patients with subarachnoid hemorrhage (Soto-Hernández et al. 1996). Nowadays, conventional angiography has been largely replaced by magnetic resonance angiography and by CT angiograms for the diagnosis of intracranial vascular lesions in patients with neurocysticercosis (Del Brutto 2008).
8.2.2 Computed Tomography (CT) and Magnetic Resonance Imaging (MRI)
Introduction of CT really improved the diagnosis and understanding of neurocysticercosis as it allowed, for the very first time, accurate preoperative visualization of cysticerci as well as proper identification of the number and topography of parasites and their stages of viability or involution (Carbajal et al. 1977; Bentson et al. 1977). CT also allowed the development of clinical classifications of neurocysticercosis based on the topography of lesions and the activity of the disease (Sotelo et al. 1985) and was important for determining the rational therapeutic approach for the different forms of the disease. Then, the advent of MRI provided a series of imaging advantages over CT, including the possibility of multi-planar (axial, coronal, and sagittal) reconstruction of images, the capacity to visualize the posterior fossa without bone artifacts, and a contrast resolution that far exceeds that of CT (Martinez et al. 1989; Suss et al. 1986). A major shortcoming of MRI, however, is failure to detect small calcifications which—in many cases—represent the sole imaging evidence of the disease. Because of this major limitation of MRI, CT remains the best screening neuroimaging procedure for patients with suspected neurocysticercosis. Other disadvantages of MRI include the scarcity of the equipment in poor regions where cysticercosis is endemic and the high costs of the exam.
8.2.2.1 Parenchymal Neurocysticercosis
The stage of viability and involution of parenchymal brain cysticerci determines their appearance on CT and MRI. Viable (vesicular) cysticerci appear as small and rounded cystic lesions that are well demarcated from the surrounding brain parenchyma. There is little or no perilesional swelling and no abnormal enhancement after contrast medium administration. Many vesicular cysts have in their interior a brightening nodule representing the scolex, giving the lesions a pathognomonic “hole-with-dot” appearance (Fig. 8.1). When the infection is massive, as in the so-called heavy non-encephalitic form of neurocysticercosis (García and Del Brutto 1999), the brain looks like a “Swiss cheese” (Fig. 8.2) or even as a “starry night” (Fig. 8.3). During the first stage of involution (colloidal stage), cysticerci appear as ill-defined lesions surrounded by edema, and most of them show an abnormal nodular or ring pattern of enhancement after contrast medium administration; the scolex is seldom visualized in colloidal cysticerci using CT or conventional MRI sequences (Fig. 8.4). Therefore, the presence of one or two ring-enhancing lesion in the brain parenchyma may represent a diagnostic challenge, as other infections or even neoplasms may present with similar neuroimaging findings. In such cases, the practice of diffusion-weighted images and apparent diffusion coefficient maps facilitates the diagnosis by allowing the recognition of the scolex (do Amaral et al. 2005) (Fig. 8.5).
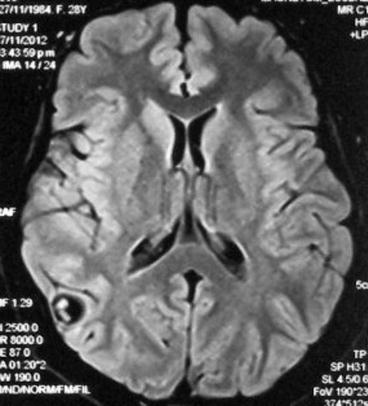
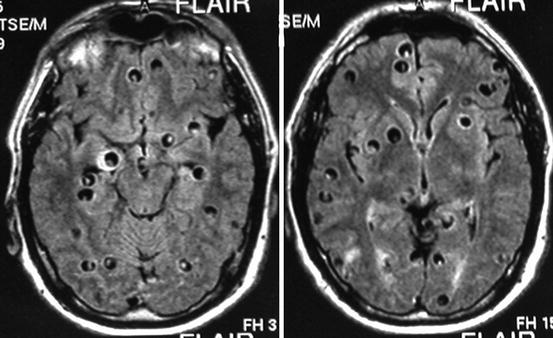
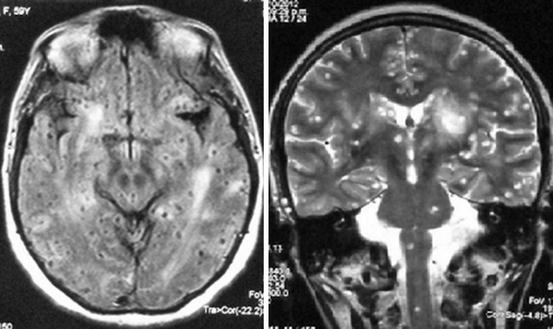
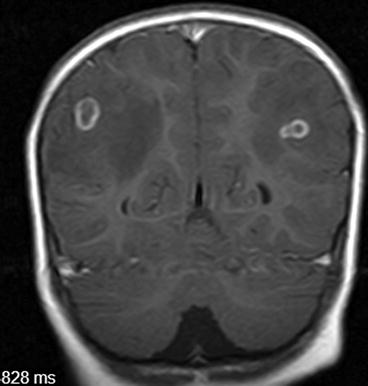
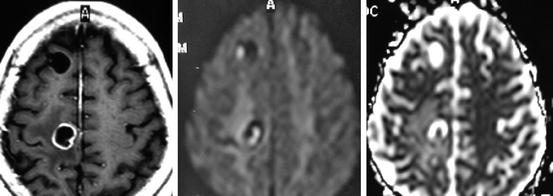

Fig. 8.1
Fluid attenuation inversion recovery MRI showing single parenchymal brain cysticerci in the vesicular stage located in the right parietal lobe. The scolex is clearly visualized within the cyst

Fig. 8.2
Contrast-enhanced MRI of a patient with heavy non-encephalitic parenchymal brain cysticercosis giving the brain a “Swiss cheese” appearance

Fig. 8.3
Fluid attenuation inversion recovery (left) and T2-weighted (right) MRI of a patient with massive infection of the brain parenchyma by small cysticerci, giving the so-called starry night effect

Fig. 8.4
Contrast-enhanced MRI showing two colloidal parenchymal brain cysticerci (Reproduced from Del Brutto (2012a), ©Oscar H. Del Brutto)

Fig. 8.5
MRI of a patient with colloidal parenchymal brain cysticerci. Scolices are not visualized on contrast-enhancing imaging (left), but are well seen in diffusion-weighted images (center) and in the apparent diffusion coefficient map (right) (Reproduced with permission from: Del Brutto (2012b))
A particular neuroimaging pattern of parenchymal neurocysticercosis in the colloidal stage is that of the so-called cysticercotic encephalitis. In this severe form of the disease, both CT and MRI show diffuse or multifocal brain edema and collapse of the ventricular system without midline shift (Del Brutto and Campos 2012). In such cases, multiple small nodular or ring-enhancing lesions appear disseminated through the brain parenchyma after contrast medium administration (Fig. 8.6).
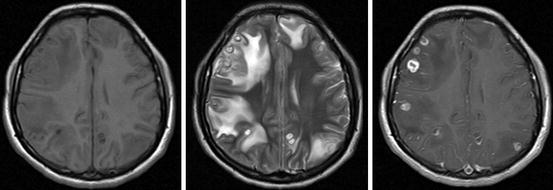

Fig. 8.6
MRI of a patient with cysticercotic encephalitis. T1-weighted image showing diffuse brain edema and collapse of the ventricular system (left), T2-weighted imaging showing multiple colloidal cysticerci surrounded by edema (center) and contrast-enhanced imaging showing ring-enhancing lesions (right) (Reproduced with permission from: Del Brutto (2012b))
Parenchymal brain cysticerci may also appear as slightly hyperdense/hyperintense nodular-enhancing lesions surrounded or not by edema or perilesional gliosis. This pattern corresponds to the granular stage of cysticerci which, as previously noted, represents degenerated cysticerci. Calcified cysticerci appear, on CT, as small hyperdense nodules not surrounded by edema or abnormal contrast enhancement. As noted before, the sensitivity of conventional MRI sequences for the detection of calcified lesions is not as good as that of CT (Fig. 8.7). There is some recent evidence, however, that use of susceptibility-weighted images may enhance the visualization of calcifications by MRI (Wu et al. 2009).
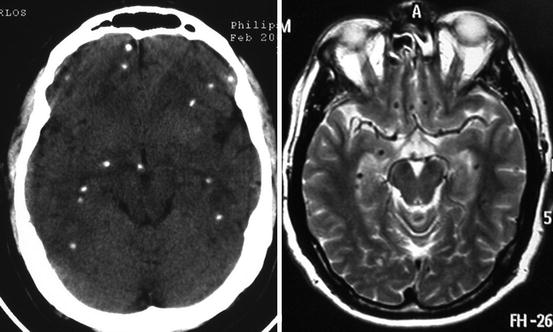

Fig. 8.7
Contrast-enhanced CT (left) and T2-weighted MRI (right) showing multiple parenchymal brain calcifications, which are more easily discernible with CT than with MRI
8.2.2.2 Subarachnoid Neurocysticercosis
Subarachnoid cysts located within cortical sulci may present with similar neuroimaging findings than those described for parenchymal brain cysts or may grow to distort the cerebral anatomy and compress neighboring structures (Fig. 8.8). Lesions located within the Sylvian fissures or at the basal CSF cisterns may have a multilobulated appearance and often attain a large size, displacing neighboring structures and behaving as space-occupying mass lesions (Fig. 8.9). Hydrocephalus, related to inflammatory occlusion of Luschka and Magendie foramina, may also be seen. The fibrous arachnoiditis that is responsible for the development of hydrocephalus is seen on neuroimaging studies as abnormal leptomeningeal enhancement at the base of the brain (Fleury et al. 2011; Kimura-Hayama et al. 2010).
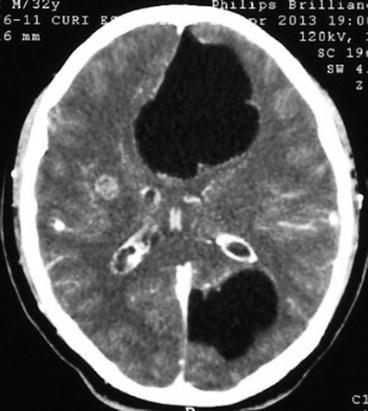
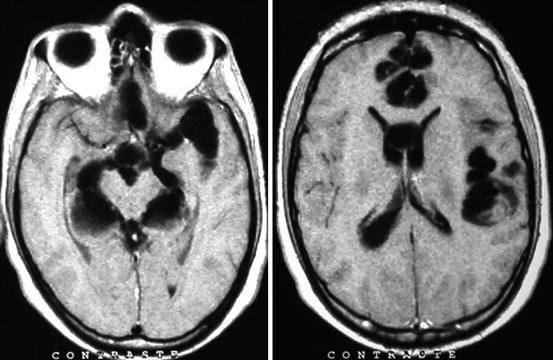

Fig. 8.8
Contrast-enhanced CT showing two large subarachnoid cysticerci arising from cortical sulci that distort brain anatomy and compress neighboring structures

Fig. 8.9
T1-weighted MRI showing clumps of subarachnoid cysticerci involving the left Sylvian fissure and the basal CSF cisterns surrounding the midbrain
Cysticerci located in the sellar region are rare. Those lesions may be confined to the intrasellar compartment or may be associated with hydrocephalus or subarachnoid cysts at the suprasellar cistern (Del Brutto et al. 1988). According to a recent review, MRI is better than CT to visualize those cysts (Del Brutto and Del Brutto 2013b).
Cerebrovascular complications of neurocysticercosis are well visualized with modern neuroimaging techniques. In patients with cysticercosis-related cerebral infarctions, the association of subarachnoid cystic lesions or abnormal enhancement of basal leptomeninges suggests the correct diagnosis (Del Brutto 2008). Magnetic resonance angiography is a valuable noninvasive imaging modality to demonstrate narrowing or occlusion of intracranial arteries in patients with subarachnoid neurocysticercosis (Fig. 8.10).
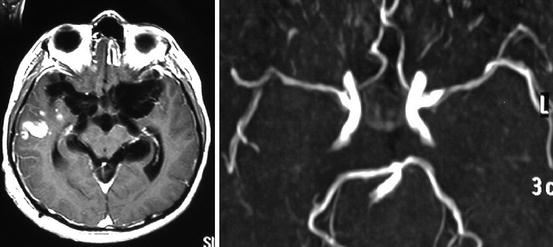

Fig. 8.10
Contrast-enhanced MRI of a patient presenting with an acute stroke syndrome, showing large clumps of cysticerci within the basal subarachnoid cisterns and a recent infarction in the right temporal lobe (left) and MRA showing multiple stenosis of major intracranial arteries arising from the circle of Willis (right)
8.2.2.3 Ventricular Neurocysticercosis
Ventricular cysticerci appear on CT as hypodense lesions that cause asymmetric obstructive hydrocephalus. Most often, ventricular cysts cannot be visualized directly as they are isodense with CSF. In contrast, ventricular cysts are readily visualized on MRI because the signal properties of the cystic fluid, the scolex, or the vesicular wall differ from those of the CSF (Kimura-Hayama et al. 2010) (Fig. 8.11). Cyst mobility within the ventricular cavities in response to movements of the head, the so-called ventricular migration sign, facilitates the diagnosis of ventricular cysticercosis in some cases. In other cases, parasitic membranes or inflammation of the ependymal lining (ventriculitis) occludes Monro’s foramina and causes asymmetric internal hydrocephalus, which is most often noticed after the placement of a ventricular shunt, as the lateral ventricle contralateral to the shunt remains dilated after the derivative procedure. A rare finding in patients with ventricular cysticercosis is the so-called double compartment hydrocephalus (DeFeo et al. 1975), in which the fourth ventricle is isolated from the rest of ventricular cavities and from the subarachnoid space due to simultaneous occlusion of the cerebral aqueduct and the foramina of Luschka and Magendie.
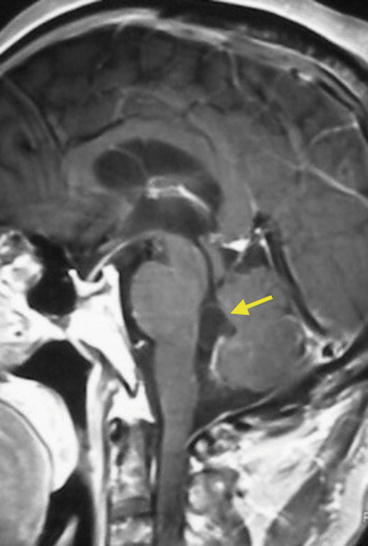

Fig. 8.11
Contrast-enhanced MRI showing cysticercus within the fourth ventricle (arrow). The vesicular wall is clearly distinguished from the CSF
8.2.2.4 Spinal Cord Neurocysticercosis
MRI has become the imaging modality of choice for the evaluation of patients with suspected cysticercosis of the spinal cord or the spinal subarachnoid space. MRI allows precise localization of intramedullary cysticerci (Homans et al. 2001; Qi et al. 2011). Those cysts are usually single and most often located at the lower segments of the thoracic spinal cord, although they may also be seen at the cervical or lumbosacral levels. Most of these cysts appear as ring- or nodular-enhancing lesions, and the scolex can be visualized in a sizable subset of cases (references). Leptomeningeal cysts are also easily identified with MRI (Fig. 8.12). These lesions may be freely mobile within the spinal subarachnoid space and may change their position during the exam according to movements of the patient in the exploration table. A recent study stresses the high frequency of spinal cysticerci among patients with intracranial subarachnoid neurocysticercosis (Callacondo et al. 2012). It is likely that this study will change the diagnostic approach of patients with intracranial subarachnoid neurocysticercosis, since all of them will probably need MRI investigation of the spine to detect hidden lesions.
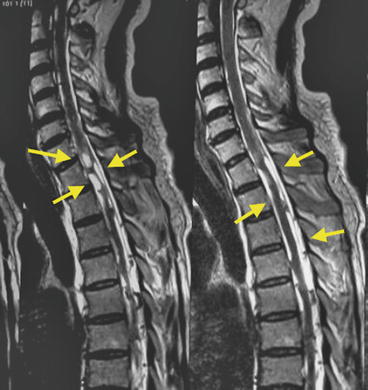

Fig. 8.12
T2-weighted MRI showing multiple spinal subarachnoid cysticerci (arrows)
8.3 Immunological Diagnosis of Neurocysticercosis
An ideal immunodiagnostic test for human neurocysticercosis should be 100 % sensitive (detecting all cases), 100 % specific (ruling out all cases without the disease), and provides information on the presence or not of living parasites as well as an idea of the parasite burden. Existing tests are far from this goal, and even if this could be achieved, immunodiagnosis would still not provide important information available from neuroimaging such as the location of lesions, degree of inflammation, and other particularities affecting the medical or surgical management. Thus, immunodiagnosis should always be interpreted on the light of clinical and neuroimaging findings in a given patient (Rodríguez et al. 2012).
8.3.1 Antibody Detection
More than 100 years ago, it was reported the detection of specific antibody reactions to cysticercal antigens by means of the complement fixation text in pig serum (Moses 1909) and in human CSF (Weinberg 1911). Along the years, a series of antibody detection techniques have been applied to the diagnosis of cysticercosis including immunofluorescence, hemagglutination, enzyme-linked immunosorbent assay (ELISA), and lately the EITB (immunoblot) (Arambulo et al. 1978; Diaz et al. 1992; Diwan et al. 1982; Larralde et al. 1986; Tsang et al. 1989).
The ELISA technique, described in 1971, did overcome previous tests because of its better performance and quantitative results. It was applied to the diagnosis of neurocysticercosis by the use of a crude cysticercal antigen since 1978 (Arambulo et al. 1978) and widely adopted a few years later. ELISA-based assays seem to perform better in CSF than in serum due to less likelihood of cross-reactions. Indeed, cross-reactions with other helminths are common, particularly with cestodes including Echinococcus spp. and Hymenolepis nana. In 1989, the EITB using a group of seven well-defined lentil-lectin purified glycoprotein antigens provided a much more sensitive and specific assay and became the test of choice for the diagnosis of cysticercosis (Tsang et al. 1989). Strong antibody reactions suggest severe infections and are infrequent in asymptomatic populations in community-based studies (Corona et al. 1986; Zini et al. 1990).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







