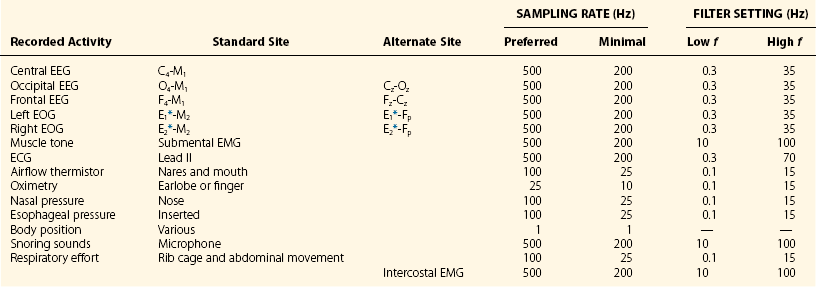
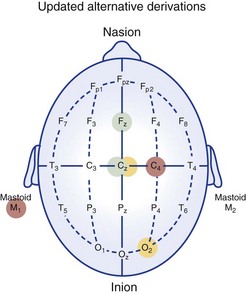
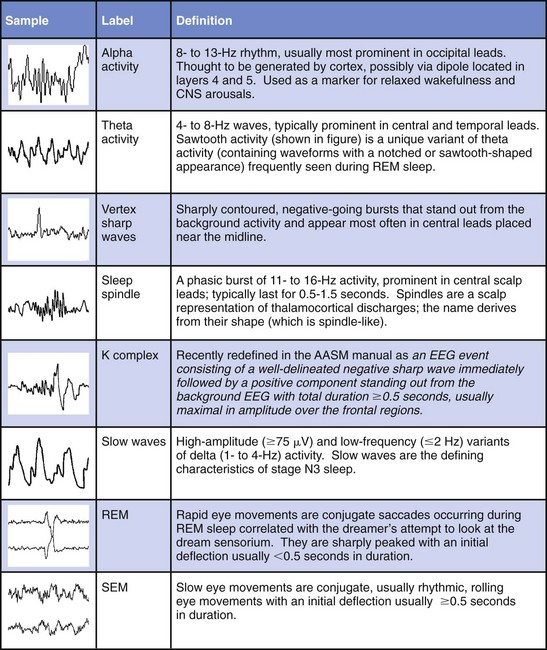
Chapter 18 For classifying sleep stages, the AASM manual recommends recording frontal, central, and occipital electroencephalograms (EEGs)—specifically, monopolar derivations from F4, C4, and O2 linked to the contralateral mastoid (M). Backup electrodes are placed at homologous sites at left scalp loci. An alternative recording montage allows substitution of midline bipolar recordings from frontal and occipital derivations (Table 18-1, Figs. 18-1 through 18-3). Figure 18-1 Classic Rechtschaffen and Kales derivations for recording the electroencephalogram. Figure 18-2 Updated American Association of Sleep Medicine (AASM) recommended derivations for recording the electroencephalogram. To detect eye movements, electrodes are placed near the eyes’ right and left outer canthi (E2 and E1, respectively). The manual recommends a monopolar electrooculographic (EOG) montage, with E2 placed 1 cm above and E1 placed 1 cm below the outer canthus. When a lateral eye movement occurs, the positive corneal potential moves toward one electrode and away from the other. Therefore this electrode arrangement produces robust right-versus-left EOG out-of-phase activity when horizontal eye movements occur. Consequently, eye movements are easily differentiated by frontal EEG activity proximal to the EOG electrodes, which appears as an in-phase signal. Staggering the electrode placements slightly above and slightly below each eye’s horizontal plane allows some limited appreciation of vertical eye movements on the PSG. An alternative recording montage that allows better visualization of vertical eye movements (albeit sacrificing the in-phase/out-of-phase differentiation by frontal EEG activity) has both EOG placements 1 cm below the outer canthi and referenced to the middle of the forehead (see Table 18-1). Sleep-stage classification involves characterizing the predominant electrocortical activity over a set length of time. This time-domain categorization proceeds for each specified time interval, called an epoch. The AASM manual standardized the epoch length at 30 seconds, which corresponds to what had been one page when paper recordings were made, with the chart drive set to 10 mm/sec. Overall, the sleep process is classified into three distinct conceptual organizational states of the central nervous system (CNS): wakefulness, rapid eye movement (REM) sleep, and non-REM (NREM) sleep. On the basis of EEG, EOG, and EMG activity, each recorded epoch is classified as either stage N1 (NREM 1), N2 (NREM 2), N3 (NREM 3), R (REM), or W (wakefulness).* Six EEG waveforms are commonly used to differentiate states and classify sleep stages: 1) alpha activity, 2) theta activity, 3) vertex sharp waves, 4) sleep spindles, 5) K-complexes, and 6) slow-wave activity (Table 18-2). In patients with well-defined alpha activity, wakefulness and sleep are easily differentiated. Stage W is scored when alpha activity occupies 15 or more seconds of an epoch (Fig. 18-4). By contrast, when clear alpha activity is absent, differentiating wakefulness from sleep can, at times, be difficult. Figure 18-4 Epoch of stage W. Vertex sharp waves often herald sleep onset and thereby provide a valuable landmark for recognizing stage N1 sleep. Wakefulness usually transitions to either stage N1 or N2. Stage N1 is marked by a general slowing of background EEG activity with the appearance of theta and vertex sharp waves. N1 may also be marked by the cessation of blinking and saccadic eye movements and by the appearance of slow eye movements (Fig. 18-5). Although not required, N1 can be discerned by its low-voltage, mixed-frequency background EEG containing theta activity in the absence of slow waves, sleep spindles, K-complexes, prominent alpha activity, or REM. Sleep stage scoring reliability for stage N1 is substantially lower than for any other stage; this holds true for both interscoring and intrascoring agreement. This problem arises from stage N1 being largely defined by rules of exclusion rather than by clearly recognizable electrophysiologic events. Luckily, stage N1 is transitory and accounts for only a small percentage of the night’s sleep complement. Figure 18-5 Epoch of stage N1. By contrast, stage N2 is easily recognized by sleep spindles or K-complexes (Fig. 18-6) that occur on a low-voltage, mixed-frequency background EEG in the absence of significant slow-wave activity (i.e., less than 6 seconds of 75 µV or more). Figure 18-6 Epoch of stage N2. When 6 or more seconds of slow-wave activity are present in an epoch, it is scored as stage N3 (Fig. 18-7). The designation N3 generally conforms to activity previously designated as slow-wave sleep because of the highly synchronized low-frequency activity. Slow-wave activity scoring requires 75 µV or greater peak-to-peak amplitude from a frontal derivation. However, it is important to realize that the recording montage dramatically affects EEG amplitude. Thus if a frontal EEG is recorded using the AASM alternate bipolar montage (Fz-Cz), amplitudes will be lower, and N3 sleep will be reduced. The AASM manual does not provide a guideline for amplitude adjustment; therefore stage N3 scoring should derive from the monopolar central lead included in the alternate montage. Figure 18-7 Epoch of stage N3. Criteria for scoring REM sleep—or stage R sleep, as it has been renamed, although most will likely continue to call it REM sleep—remained essentially unchanged. Stage R is characterized by low-voltage, mixed-frequency EEG, very low chin EMG levels, and REM (Fig. 18-8). Sawtooth activity represents a unique variant of theta activity, containing waveforms with a notched or sawtooth-shaped appearance, frequently observed during stage R (Table 18-3). Table 18-3 Electrophysiologic Characteristics of Each Sleep Stage EEG, electroencephalogram; EMG, electromyogram; s, seconds. †Special form of theta wave called sawtooth owing to its appearance. ‡For purposes of sleep staging, monopolar slow-wave activity from frontal or central derivations must be ≥75 µV. Figure 18-8 Phasic and tonic rapid eye movement (REM) sleep. When an epoch contains characteristic features for more than one sleep stage, it is scored according to the characteristics that make up its majority. One source of scoring difficulty arises when epochs without actual eye movements are contiguous with REM sleep. The AASM manual does not address the concept of differentiation between phasic and tonic REM sleep (see Fig. 18-8). Nevertheless, this concept is widely used in research and can provide useful instruction in this matter. The term phasic REM sleep refers to epochs scored as stage R clearly accompanied by eye movements and sometimes by other phasic events. By contrast, an epoch designated as tonic REM sleep has the same background EEG activity, nearly completely diminished chin EMG, but lacks eye movements. If such an epoch occurred in isolation, it would likely be scored as N1; however, when surrounded by epochs of phasic REM sleep, it is considered stage R. In fact, N1-like epochs without eye movement contiguous with REM sleep continue to be scored as stage R until some indication occurs that another sleep process has emerged. Indicators can be a spindle, a K-complex, a CNS arousal, an increase in chin EMG, a body movement followed by slow eye movements, or the appearance of another scorable sleep stage in the first half of the epoch. The AASM manual provides examples to guide scoring decisions at stage transitions. CNS arousals involve an abrupt shift during sleep to faster EEG activities—including theta, alpha, and beta but not sleep spindles—for 3 seconds or longer. CNS arousal scoring rules largely derive from a previous concept called EEG speeding. Usually, the frequency shift manifests as alpha activity and is best visualized in occipital leads. For the shift to qualify as an arousal, the person must have been asleep for at least 10 seconds. Finally, during REM sleep, the EEG shift must be accompanied by at least 1 second of increased chin EMG tone (Fig. 18-9) because alpha bursts routinely appear during REM sleep and do not intrinsically represent pathophysiology. The 3-second duration was not arbitrary; it was the minimum arousal duration reliably scored by hand among the task force members. Undoubtedly, digital systems could reliably score shorter arousals, but the clinical significance of such events is not known. Figure 18-9 Central nervous system arousals. The cyclic alternating pattern (CAP) refers to an EEG pattern seen during sleep (Fig. 18-10) in which EEG activity bursts—usually a high-amplitude slow, sharp, or polymorphic wave burst—alternate with periods of quiescence. The burst constitutes the A phase, and the quiescence is labeled the B phase. The Parma Groups, led by Terzano and Parrino, organized an international workshop during which recording and scoring techniques were codified. In essence, the A phase was subcategorized into three types based on the presence and duration of intermixed or intermingled EEG alpha activity. Type A1 contains little or no alpha EEG activity and consequently does not meet AASM arousal scoring criteria. Type A2 often meets AASM arousal criteria, and A3 meets AASM criteria 95% of the time. Sleep associated with A1 bursts is conceptualized as unstable but is preserved by a descending cortical response that helps reinforce a protective thalamic gating mechanism. By contrast, if the attempt to reinforce this gate fails, an arousal or awakening occurs, and an A3 burst is observed. A2 responses fall somewhere in between (Fig. 18-11). Figure 18-10 Cyclic alternating pattern (CAP). Figure 18-11 Examples of cyclic alternating pattern A phase types 1, 2, and 3 activity. Key features needed to assess SDB include airflow, respiratory effort, blood oxygenation, and sleep disturbance. The AASM manual recommends recording the four data channels when clinically evaluating adult patients with overnight PSG (see Table 18-1 for sampling rates and filter settings). This is done with 1) a thermal sensor at the nose and mouth to detect apnea; 2) a nasal pressure transducer to detect hypopnea; 3) either an esophageal manometer or chest/abdominal inductance plethysmograph—or, alternatively, intercostal EMG—to detect respiratory effort; and 4) a pulse oximeter with its signal averaged over 3 seconds or less.
Diagnostic Assessment Methods in Adults
Sleep Staging
Recording
Electroencephalogram for Brain Activity
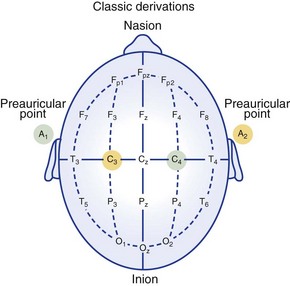
Electrode locations as recommended in 1969. Electrode pairs are indicated by the same colors.
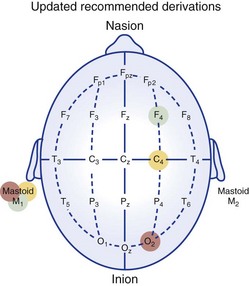
Electrode locations as recommended in 2007 by AASM.
Eye Movements
Staging
Classification
Waves
Stages
Stage W.
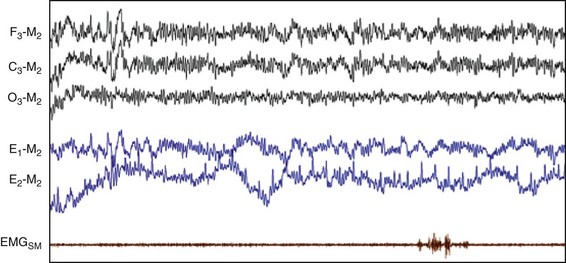
This figure depicts 30 seconds of polysomnographic activity characterizing stage W (wakefulness) according to AASM scoring criteria. C3, left central scalp derivation; E1, left outer canthus eye electrode location; E2, right outer canthus eye electrode location; EMGSM, surface electrode electromyogram (EMG) from submentalis muscle; F3, left frontal scalp derivation; M2, right mastoid electrode location; O3, left occipital scalp derivation.
Stage N1.
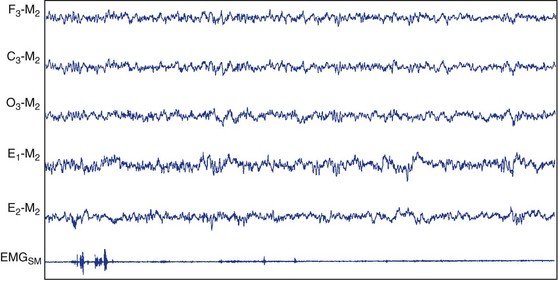
This figure depicts 30 seconds of PSG activity characterizing stage N1 sleep according to AASM scoring criteria. C3, left central scalp derivation; E1, left outer canthus eye electrode location; E2, right outer canthus eye electrode location; EMGSM, surface electrode EMG from submentalis muscle; F3, left frontal scalp derivation; M2, right mastoid electrode location; O3, left occipital scalp derivation.
Stage N2.
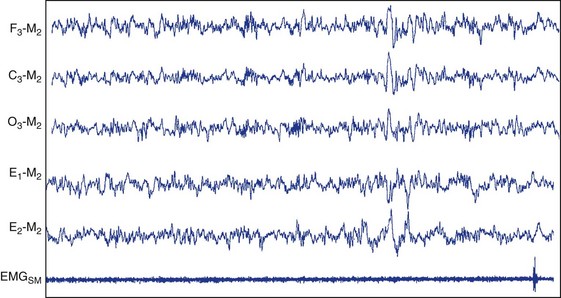
This figure depicts 30 seconds of PSG activity characterizing stage N2 sleep according to AASM scoring criteria. C3, left central scalp derivation; E1, left outer canthus eye electrode location; E2, right outer canthus eye electrode location; EMGSM, surface electrode EMG from submentalis muscle; F3, left frontal scalp derivation; M2, right mastoid electrode location; O3, left occipital scalp derivation.
Stage N3.
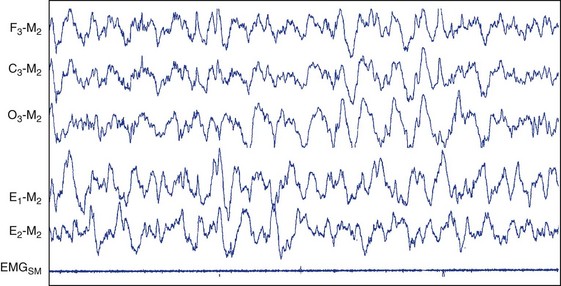
This figure depicts 30 seconds of PSG activity characterizing stage N3 sleep according to AASM scoring criteria. C3, left central scalp derivation; E1, left outer canthus eye electrode location; E2, right outer canthus eye electrode location; EMGSM, surface electrode EMG from submentalis muscle; F3, left frontal scalp derivation; M2, right mastoid electrode location; O3, left occipital scalp derivation.
Stage R.
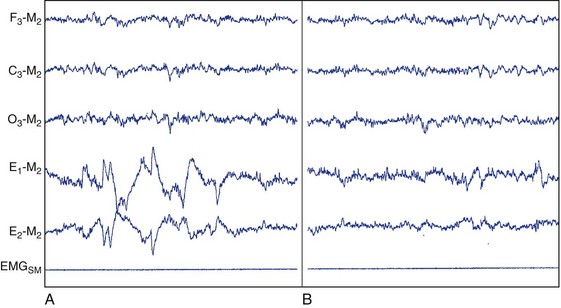
This figure depicts PSG activity characterizing stage R sleep (REM sleep) according to AASM scoring criteria. A, Stage R, with phasic bursts of REM (phasic REM). B, A quiescent period of stage R sleep (tonic REM). C3, left central scalp derivation; E1, left outer canthus eye electrode location; E2, right outer canthus eye electrode location; EMGSM, surface electrode EMG from submentalis muscle; F3, left frontal scalp derivation; M2, right mastoid electrode location; O3, left occipital scalp derivation.
Smoothing Rules
Arousal Scoring
Electroencephalogram Speeding and Central Nervous System Arousals
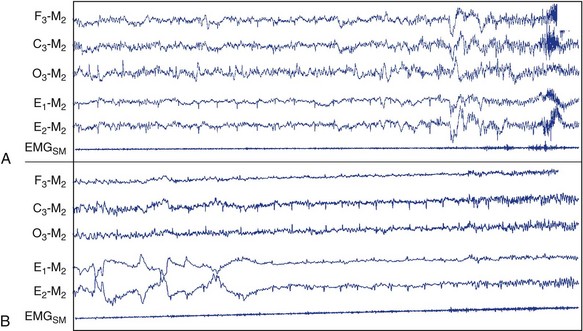
Central nervous system (CNS) arousals from non-REM (A) and REM sleep (B). C3, left central scalp derivation; E1, left outer canthus eye electrode location; E2, right outer canthus eye electrode location; EMGSM, surface electrode EMG from submentalis muscle; F3, left frontal scalp derivation; M2, right mastoid electrode location; O3, left occipital scalp derivation.
Cyclic Alternating Pattern
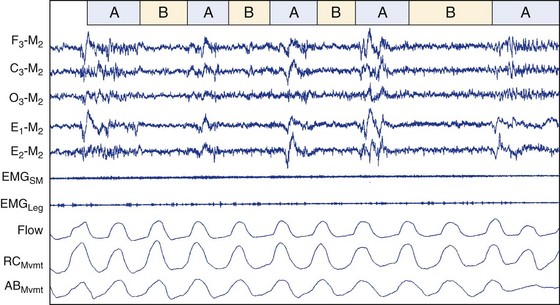
This figure depicts polysomnographic activity characterizing phase A (burst) and phase B (quiescent) activity. This period of CAP activity appears not to be associated with either periodic leg movements or breathing abnormalities. ABMvmt, abdominal movement measured with inductive plethysmography; C3, left central scalp derivation; E1, left outer canthus eye electrode location; E2, right outer canthus eye electrode location; EMGLeg, surface electrode EMG from left and right anterior tibialis; EMGSM, surface electrode EMG from submentalis muscle; F3, left frontal scalp derivation; Flow, airflow measured with nasal-oral thermistors; M2, right mastoid electrode location; O3, left occipital scalp derivation; RCMvmt, rib cage movement measured with inductive plethysmography.
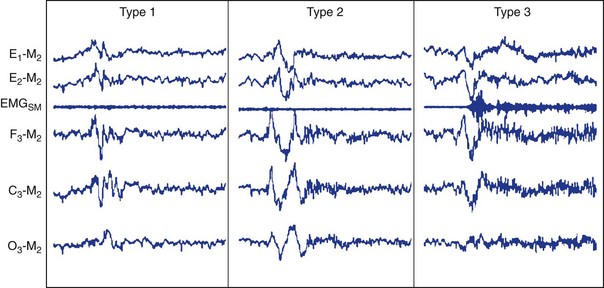
Type 1 does not appear to have EEG activity suggestive of central nervous system (CNS) arousal. By contrast, type 2 activity suggests possible arousal, whereas type 3 meets AASM criteria for CNS arousal from NREM sleep.
Sleep-Related Breathing Disorders
Recording Technique
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree