General sleep assessments
Sleep diary
SF-12, SF-36
Daytime sleepiness
Epworth Sleepiness Scale [34]
Stanford Sleepiness Scale [35]
Functional Outcomes of Sleep Questionnaire [36]
OSA
Berlin Questionnaire [37]
STOP-BANG Questionnaire [38]
Table 2.2
Internet-based locations of some sleep disorder scales
Epworth Sleepiness Scale | |
Stanford Sleepiness Scale | |
Berlin Questionnaire | |
STOP-BANG Questionnaire | |
Sleep diary |
General Sleep
Patients are often referred to sleep disorder centers with a suspicion of a rare sleep disorder, such as narcolepsy, due to their EDS, when in fact a more commonplace issue is to blame. Clinical history taking is paramount, in order to best distinguish amongst the potential causes of hypersomnia. General sleep questionnaires can then help guide the clinician in understanding symptom patterns and severity.
After an initial visit with the clinician, most patients complaining of hypersomnia should be given a sleep diary to complete. The sleep diary is an instrument in which the patient would keep track their daily sleep patterns. Information may include medications taken, bedtime, time to sleep onset, number of awakenings, time of waking, time out of bed, and length and timing of any naps. A sleep diary should typically be used for 1–2 weeks, but it may also be used as a long-term method of assessing a patient’s sleep time, particularly as behaviorally induced insufficient sleep is a common cause of daytime sleepiness. It is worth considering that insufficient sleep may be quite subtle; a loss of 30–60 min of sleep per weekday may induce chronic mild sleep deprivation, from which some patients may be clearly symptomatic. A sample sleep diary is provided in Fig. 2.1; another example is available on the Internet, referenced in Table 2.2.


Fig. 2.1
Sample sleep log
A nonspecific assessment of the effect of sleep disorders on the lives of patients may be performed by the Short Form 36 (SF-36), a 36-question survey with “an 8-scale profile of functional health and well-being scores as well as psychometrically-based physical and mental health summary measures and a preference-based health utility index” [3]. This questionnaire has been used to assess many medical disorders, including many sleep disorders. The SF-12, a shorter, one-page form, is also available and has been used in sleep research [4].
Excessive Daytime Sleepiness
The most widely used questionnaire specifically for daytime sleepiness is the Epworth Sleepiness Scale (ESS), developed in 1991 by Dr. Murray Johns. In research studies, the ESS has been shown to be reliable when testing and retesting individuals over time. The ESS assesses a patient’s self-report of sleepiness by asking about eight different situations and the likelihood of a patient dozing in each of them. The Likert response scale ranges from 0 (“would never doze”) to 3 (“high chance of dozing”). Adding the results from each question determines a total score that measures subjective sleep propensity “in recent times.” Early research demonstrated that ESS scores greater than 15 were observed in patients with narcolepsy, idiopathic hypersomnolence, or moderate/severe OSA. Since the ESS is easy to use, it has been commonly used in research studies; however conflicting results exist regarding the ability of the ESS to predict objective measures of daytime sleepiness [5, 6]. These results suggest that in practice, the ESS is best used to assess subjective sleepiness in a standardized manner though it is unlikely to replace objective testing. Tracking ESS scores longitudinally for individual patients does appear useful in assessing change or treatment response over weeks to months.
The Stanford Sleepiness Scale (SSS) provides an instantaneous subjective measure of sleepiness as a single question on a seven-point scale [7, 8]. The scale ranges from “feeling active, vital, alert, or wide awake” (1 on the scale) to “no longer fighting sleep, sleep onset soon, or having dreamlike thoughts” (7 on the scale). This scale, in contrast to the ESS, can be used by the same patient many times in 1 day to help track sleep drive. However, this scale becomes less meaningful over longer time periods (weeks to months), since many factors can impact an instantaneous measure of daytime sleepiness. The Karolinska Sleepiness Scale (KSS) is very similarly designed, with the primary difference being that it has a nine-point scale instead of seven [9]. Both of these immediate-assessment sleep scales are validated with objective sleep measures including EEG and performance [10, 11].
The impact of daytime sleepiness on activities of daily living can be assessed by the Functional Outcomes of Sleep Questionnaire (FOSQ). The FOSQ was designed at a fifth grade reading level, is designed to take 15 min to complete, and contains 74 questions over six domains (orientation, physical independence, mobility, occupation, social integration, and economic self-sufficiency), as well as assessment of several additional daily endeavors potentially affected by daytime sleepiness. Initially, this questionnaire was validated to discriminate between normal subjects and those seeking medical attention for a sleep problem [12]. It has since been shown to change with positive pressure therapy for OSA [13], treatment with modafinil [14], and with other treatments of OSA [15]. A modified short form of the FOSQ, the FOSQ-10, has also been validated and may be easier for clinical use in following patients over time [16].
Obstructive Sleep Apnea
OSA is a disorder of repetitive collapse of the upper airway causing oxygen desaturations and electroencephalographic arousals. Fairly common in the general population (4 % of middle-aged men and 2 % of middle-aged women based on one study) [17], OSA has been demonstrated to increase risk for developing hypertension, heart disease, and cardiovascular disease [18]. Primary symptoms of OSA include snoring, sleep disruption, and most relevant to this chapter, EDS.
The Berlin Questionnaire is one of the more frequently used clinical screening tools for the assessment of OSA: The Berlin Questionnaire was an outcome of the Conference on Sleep in Primary Care, which involved 120 U.S. and German pulmonary and primary care physicians and was held in April 1996 in Berlin, Germany. Questions were selected from the literature to elicit factors or behaviors that, across studies, consistently predicted the presence of sleep–disordered breathing [19]. The questionnaire has three sections. Section one evaluates symptoms including snoring and witnessed apneas, section two covers daytime sleepiness severity, and section three assesses the presence of hypertension and calculates a body mass index. In section 1, high risk was defined as persistent symptoms (3–4 times/week) in two or more questions about their snoring. In section 2, high risk was defined as persistent (3–4 times/week) sleepiness. In section 3, high risk was defined as a history of high blood pressure or a body mass index (BMI) more than 30 kg/m2. When two of the three sections meet the criteria for high risk, the patient is at high risk for OSA [20]. While many questionnaires are completed solely by the patient, this questionnaire may require clinician input for the BMI calculation or to supply blood pressure status.
Initially, the STOP-BANG Questionnaire was developed as a short preoperative assessment used by anesthesiologists to screen for OSA. The tool covers similar content as the Berlin Questionnaire, containing eight questions in two sections (the tool was originally designed as STOP only; BANG was added at a later date in an attempt to improve specificity). The name of the questionnaire is developed from the primary content of each question, including s noring, t iredness, o bserved apneas, blood p ressure, BMI, a ge, n eck circumference, and g ender. While the questions on snoring, tiredness, apneas, high blood pressure, age (over 50 years) and gender are straightforward for patients to complete, questions on BMI (more than 35 kg/m2) and neck circumference (greater than 40 cm) often require clinician interaction. A positive answer to three or more questions gives a high probability of OSA [21]. Thus, the STOP-BANG tool is easier to score than the Berlin Questionnaire, but similarly requires some clinical measurements.
Objective Testing
While subjective testing for sleep disorders has the advantages of being quick and inexpensive, objective testing is generally considered to be the gold standard. Objective testing removes the possibility of reporting biases and allows for an improved assessment of the patient’s symptom severity. Objective testing of EDS may include overnight polysomnography, out-of-center sleep tests, multiple sleep latency tests (MSLTs), maintenance of wakefulness tests (MWTs), and actigraphy.
Overnight In-Laboratory Polysomnography
The polysomnogram (PSG) is considered the gold standard for objective testing of sleep, evaluating electroencephalography, respiratory parameters, and muscle activity during sleep. Current guidelines from the American Academy of Sleep Medicine (AASM) recommend that polysomnography assess the following parameters: electroencephalography (EEG), eye movements (EOG), chin and leg motor activity (EMG), airflow parameters (typically nasal pressure transducer and oronasal thermistor), respiratory effort parameters (both thoracic and abdominal), oxygen saturation, and body position (Fig. 2.2) [22]. Laboratories performing sleep studies should be accredited by one of several agencies (the Joint Commission, the American Academy of Sleep Medicine, etc.) to ensure that quality metrics are being upheld. A technologist places all of the appropriate probes and wires on the patient prior to the study initiation and observes the patient throughout the night, ensuring that the patient is medically stable and that the recorded data is accurately obtained.
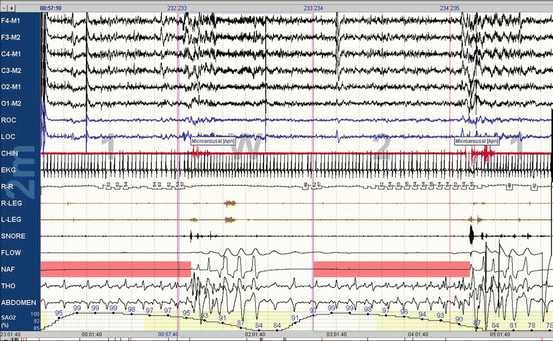

Fig. 2.2
In-laboratory polysomnography. This is a 2-min epoch of in-laboratory polysomnography (Nihon Kohden) from a 55-year-old man with obstructive sleep apnea. The top six leads are EEG (right and left frontal, central, and occipital), followed by two eye leads (right and left), the chin lead, ECG with heart rate below (R-R), two leg leads (right and left), snore channel, oronasal thermistor, nasal pressure transducer, effort bands (thorax and abdomen), and oxygen associated. Two obstructive apneas are observed at the boxes in the NAF (nasal airflow) signal with absent nasal–oral airflow and continued respiratory effort. The respiratory events are associated with increased frequency signal (arousals) in the EEG signals and oxygen desaturations
Commonly, patients are sent for in-laboratory polysomnography for evaluation of OSA, treatment of OSA with positive pressure therapy, or evaluation of treatment for OSA, such as weight loss, oral appliances, or surgical intervention. Other reasons for polysomnography may include evaluation for periodic limb movements during sleep, assessment of dangerous parasomnias, and differentiation of seizures and parasomnias. Patients with insomnia or restless leg syndrome are not typically evaluated with polysomnography, unless clinical evaluation suggests a comorbid sleep disorder. A full list of reasons to obtain (or not to obtain) an in-laboratory sleep study from the AASM practice parameters for polysomnography is supplied in Table 2.3.
Table 2.3
Practice parameters for polysomnography, 2005
Polysomnography is routinely indicated for: |
The diagnosis of sleep-related breathing disorders (standard) |
Positive airway pressure (PAP) titration in patients with sleep-related breathing disorders (standard) |
A preoperative clinical evaluation to evaluate for the presence of OSA in patients before they undergo upper airway surgery for snoring or obstructive sleep apnea (standard) |
The assessment of treatment results in the following circumstances (standard): |
1. After good clinical response to oral appliance treatment in patients with moderate to severe OSA |
2. After surgical treatment of patients with moderate to severe OSA |
3. After surgical or dental treatment of patients with SRBDs whose symptoms return |
The assessment of treatment results in the following circumstances (standard): |
1. After substantial weight loss or gain (e.g., 10 % of body weight) has occurred in patients on continuous positive airway pressure (CPAP) for the treatment of SRBDs for potential adjustment of PAP pressures |
2. When clinical response is insufficient or when symptoms return. In these circumstances, testing should be devised with consideration that a concurrent sleep disorder may be present (e.g., OSA and narcolepsy) |
Patients with heart failure, if they have nocturnal symptoms suggestive of sleep-related breathing disorders (disturbed sleep, nocturnal dyspnea, snoring) or if they remain symptomatic despite optimal medical management (standard) |
Patients with coronary artery disease, if there is suspicion of sleep apnea (guideline) |
Patients with history of stroke or transient ischemic attacks, if there is suspicion of sleep apnea (guideline) |
Patients with significant tachyarrhythmias or bradyarrhythmias, if there is suspicion of sleep apnea (guideline) |
Patients with neuromuscular disorders and sleep-related symptoms for the evaluation of symptoms of sleep disorders beyond the sleep history (standard) |
Patients with suspected narcolepsy in combination with a multiple sleep latency test (standard) |
Diagnosis of paroxysmal arousals or other sleep disruptions that are thought to be seizure related when the initial clinical evaluation and results of a standard EEG are inconclusive, with additional EEG derivations in an extended bilateral montage, and video recording is recommended in addition to standard leads (option)
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|


