Fig. 10.1
Sag. CT (a, b): ankylosing spondylitis with “bamboo spine” and fusion of joints
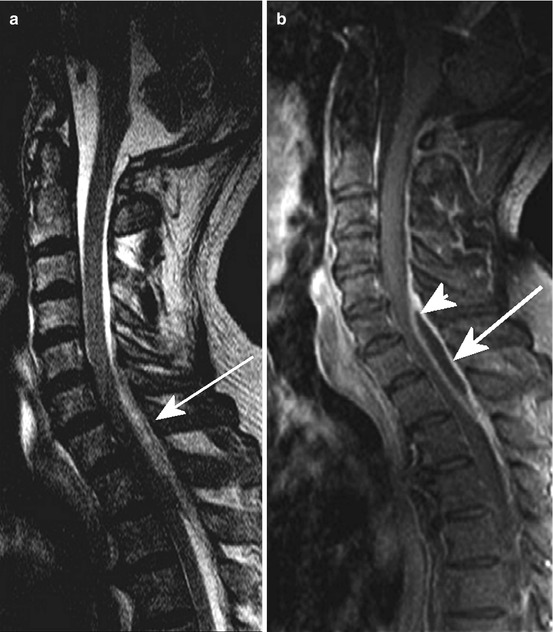
Fig. 10.2
Epidural abscess dorsal accentuated C5/6 to Th2. Sag. T2 WI (a) and T1 WI pc (b) exhibiting epidural liquid lesion (arrow) with marginal enhancement (b, arrowhead). The 67-year-old man suffered from severe neck pain for 4 days and dysaesthsia in both hands; C reactive protein elevated, leucocytosis; orthopaedic outdoor examination was stated normal 2 days before
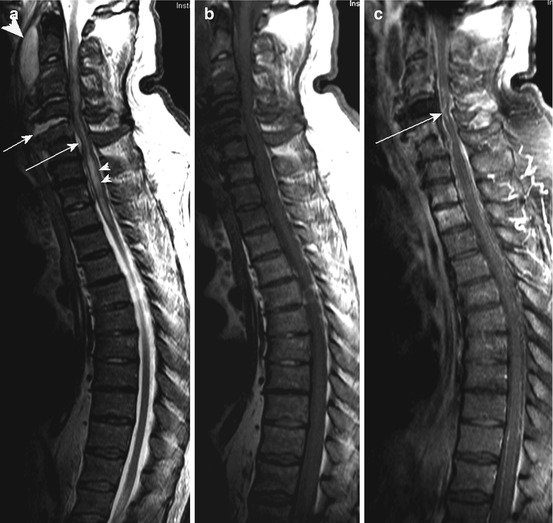
Fig. 10.3
Sag. T2 WI (a), T1 WI (b) and T1 WI fat sat pc (c) showing severe multisegmental spondylodiscitis (a, short arrow) with epidural abscess/empyema (a, long arrow and severe myelomalacia with intramedullary signal conversion (a, small arrow heads), caused by prevertebral abscess (a, large arrow head). Wallpaper-like pial and epidural enhancement, partially intramedullary (c, arrow), also enhancement of vertebral structures
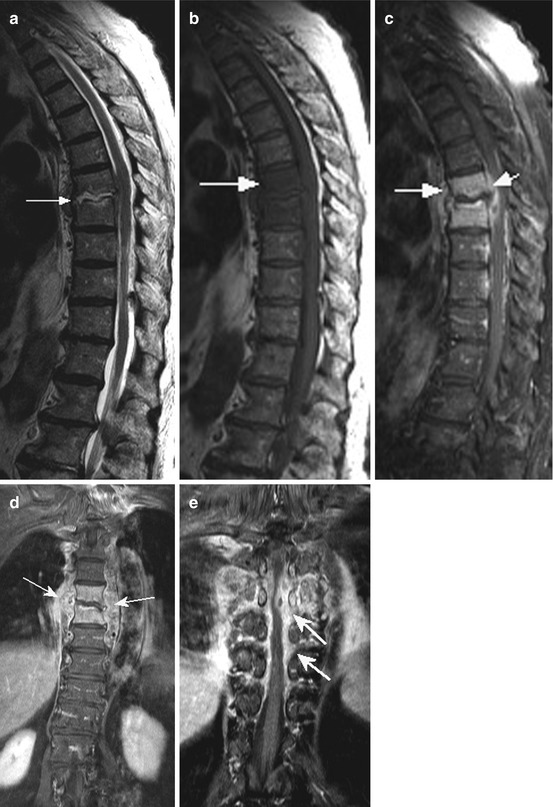
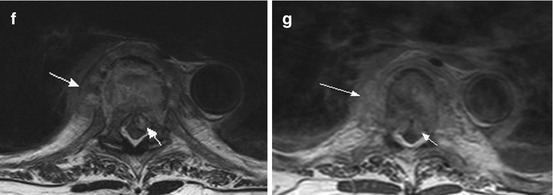
Fig. 10.4
A 91-year-old woman suffering from severe back pain and progressive paraparesis caused by spondylodiscitis at the level T6/7 with epidural abscess. Sagittal T2 WI (a) showing hyperintense signal changes of the intervertebral disc (arrow); sag. T1 WI (b) with signal decrease of the vertebral bodies Th6 and Th7 (b, arrow) and homogeneous enhancement (c, arrow; pc T1 WI); note additional enhancing masses preavertebral and epidural (c, short arrow). (d, e) (pc T1 WI coronal) exhibiting extended paravertebral inflammatory masses (d, arrows) with diffuse infiltration of the intervertebral foramina (e, arrows). (f) (T2 WI ax.) and (g) (pc T1WI ax.) revealing thickened paravertebral space (arrow) and epidural abscess with myelon compression (short arrow); note additional pleural fluid collection
10.1.2 Radicular Pain
Result from mechanical (i.e. compression or stretching), inflammatory or metabolic irritation of the nerve root within the spinal canal or the region of the intervertebral foramen (Fig. 10.5). The pain has a sharp character and radiates into the dermatome including the peripheral parts accordingly to the segmental innervation of the nerve root. When mechanical aetiology is present, e.g. intervertebral disc herniation or stenosis of intervertebral foramen, coughing, sneezing and jugular vein compression (Valsalva manoeuvre) could evoke or increase radicular pain caused by nerve root shifting.
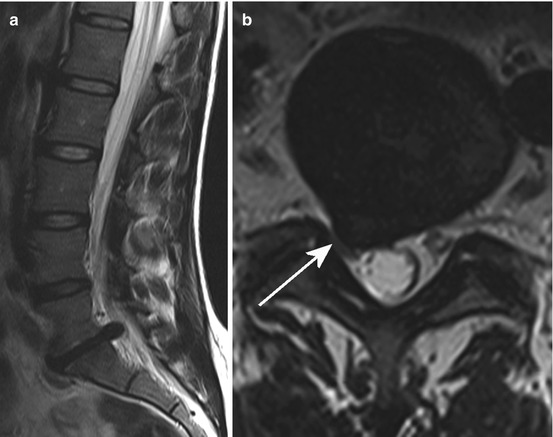

Fig. 10.5
Sag. (a) and ax. (b) T2 WI showing a mediolateral disc herniation at the level L5/S1 right in a 35-year-old woman suffering from an S1 syndrome right sided. The S1 nerve root is stretched and shifted and in addition there is a nerve root compression against ligamentous and articular structures (arrow)
Clinical examination using “straight leg raising” (Lasègue manoeuvre) shows pain exacerbation, when there is L4, L5 or S1 nerve root entrapment caused by nerve stretching. “Straight leg raising” is often restricted to between 20 and 30° in severe sciatica. Inverse Lasègue manoeuvre in a prone position with thigh extension is positive in L3 nerve root entrapment. A crossed positive Lasègue manoeuvre with pain radiation into the contralateral leg is indicative of a ruptured disc prolapse with sequestration (Fig. 10.6). Additional neurological findings may be segmental sensory and motor failures and lowered or extinct muscle jerks. Lateral foraminal or extraforaminal disc herniation in particular often also show a vegetative pain component with a deep gnawing and burning character caused by additional affection of the spinal nerve ganglion (syn.: dorsal root ganglion).
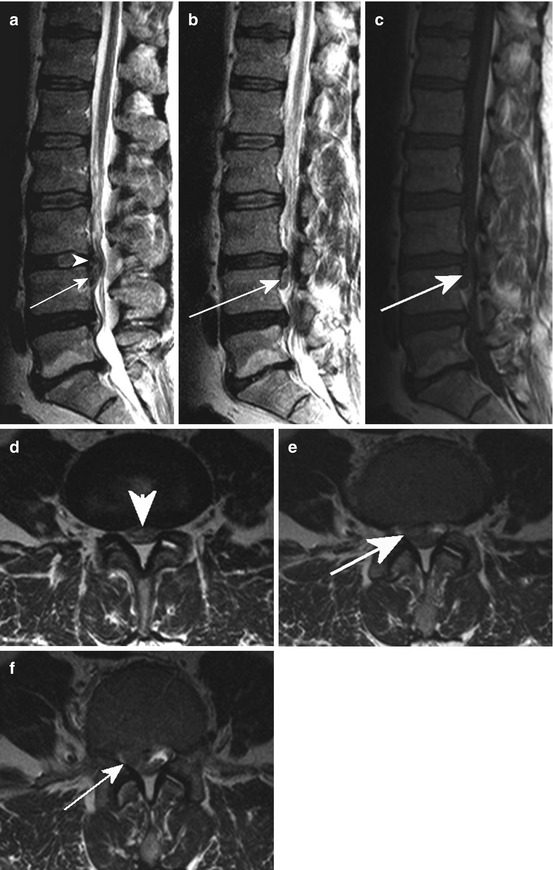

Fig. 10.6
Sag. T2 WI (a, b) and T1 WI (c) revealing a medial and mediolateral disc prolapse (arrowhead; a, d) in L3/4 with a caudal sequestration (arrow); (d–f) ax. T2 WI showing the sequestrated material within the lateral recessus (arrow)
In cervical disc herniation, downward pressure on the head in the hyperextended position of the cervical spine as well as bending to the affected side could increase radicular pain.
10.1.3 Referred Pain
Referred pain from the lower back (syn: pseudoradicular pain) originates from the above-mentioned spinal structures (i.e. the periosteum of the vertebra, the capsule of the articular facets, the outer layers of the anulus fibrosus and the intraspinal ligaments, e.g. yellow ligament and posterior longitudinal ligament) at a particular level and, in contrast to the radicular (nerve root) affection, this pain does not project much below the knees. Pain from the lower spine is often projected into the lower buttocks and posterior thighs. This is because of the activation of the same pool of segmental intraspinal neurons. Therefore, differentiation of the pain origin coming from lower spinal nerves or from the sinuvertebral nerves is sometimes impossible for the patients. However, there are no (segmental) sensory or motor deficits and deep tendon reflexes and the H reflex (e.g. S1 root) are normal. This underlines the need for exact neurological assessments including a precise anamnesis of pain evolution and evaluation of triggering manoeuvres. Besides imaging, electrophysiological investigations including nerve conduction studies and electromyography (EMG) can also help to differentiate pseudoradicular from radicular pain.
On the other hand, referred pain from visceral disease may be a challenge. Beside diseases in the pelvic, abdominal and thoracic viscera, including malignant, inflammatory and metabolic aetiologies and retroperitoneal neoplasms, infections and also (dissecting) aortal aneurysm may generate pain localized in the corresponding surface of the body (“Head zones”) region because of the above-mentioned convergence of the same intraspinal neuron pool.
10.1.4 Pain Caused by Muscle Spasm
This is often induced by local pain with reflexive muscle contraction attempting to stabilize the affected structure/segment, e.g. facet joint. Medical treatment with muscle relaxants and additional physical therapy to interrupt this circle is therefore an important part of the therapeutic concept.
Besides a wide range of other pain entities including postural back pain and sacroiliac strain, psychiatric diseases should also be considered when careful neurological and radiological investigation rule out an organic origin.
10.2 Radicular Compression Syndromes
10.2.1 Pathophysiological and Clinical Aspects
Segmental anterior and posterior roots run in their intradural sleeve through the lateral recessus and constitute the spinal nerve, which exits through the intervertebral foramen. Whereas the efferent motoric fibres of the spinal nerve derive from the lower motor neurons in the anterior horns of the grey matter of the spinal cord, the sensory afferent fibres derive from the spinal ganglion (dorsal root ganglion) at the level of the intervertebral foramen (see also Chap. 2). Both nerve roots also content visceral autonomic fibres and there are segmental connections to the sympathetic chain and their ganglia via grey and white rami communicantes. After a short distance at the exit of the intervertebral foramen, the extradural spinal nerve divides into a ramus anterior and a ramus posterior.
Radicular compression syndromes are a composition of motoric, sensoric and vegetative segmental disturbances in the corresponding myotome and dermatome. Sometimes even a sclerotome giving irradiation of pain to structures of the pelvis is mentioned. However, because of different myelin sheets, the afferent sensoric fibres are first affected with the clinical phenomena of (segmental) radicular pain and subsequent sensory deficits such as dys- and/or hypaesthesia. In addition, segmental monosynaptic deep tendon reflex via afferent spindle fibres is extenuated or extinct, especially in comparison with the healthy contralateral side. Progressive radicular compression causes additional impairment of the motoric fibres, which have thicker myelin sheets. Partial paresis or, in extremes, paralysis is flaccid and, without radicular decompression, muscle atrophy with permanent lower motor neuron palsy follows.
There is often a typical time course for radicular compression syndromes. Declining radicular pain in the presence of progressive flaccid segmental palsy in the key muscles is a red flag and should initiate emergency diagnostic clarification with a neurological examination and additional spinal imaging on the same day. This course of the disease may represent at least a partial root death and delayed therapy, i.e. neurosurgical decompression often results in partially irreversible radicular deficits. However, the correlation of clinical symptoms and neuroradiological features with clearly visible nerve root compression is essential for neurosurgical therapy. If there are any doubts about the mechanical aetiology of radicular symptoms, other causes of radicular diseases such as inflammatory aetiologies (radiculitis, e.g. borreliosis or herpes zoster; see also Chaps. 3 and 15) as well as metabolic disturbances (e.g. diabetic radiculopathy) or peripheral nerve damage (e.g. diabetic mononeuropathy) should be ruled out by additional cerebrospinal fluid (CSF) analysis, nerve conduction studies and electromyographic (EMG) investigations.
Cervical disc herniations most often occur in the segments C5/C6 and C6/C7, and lumbar prolapses in the segments L4/L5 and L5/S1 (Fig. 10.5).
Compression of sacral nerve roots may cause additional bladder dysfunction. Whereas radicular symptoms often caused by mediolateral, lateral foraminal or extraforaminal disc herniations (Figs. 10.5 and 10.6) and/or stenosis of the intervertebral foramen, medial (or central) lumbar/lumbosacral prolapse (Figs. 10.7 and 10.8) may cause a cauda equine syndrome. Lesions of the cauda equine may cause with bilateral possibly asymmetric flaccid paresis of the legs, lowered or extinct deep tendon reflexes, sphincteric disorders with disturbance of bladder and bowel function as well as polyradicular sensory loss. With lower motor neuron lesion and intact corticospinal tract, the Babinski sign is negative. Hypaesthesia or anaesthesia in the sacral dermatomes cause so-called “breeches anaesthesia”. Equivalent to acute transverse spinal cord syndromes, the acute cauda equine syndrome is an emergency case and immediately diagnostic investigation is necessary, in which initial spinal magnetic resonance imaging (MRI) is the method of choice.
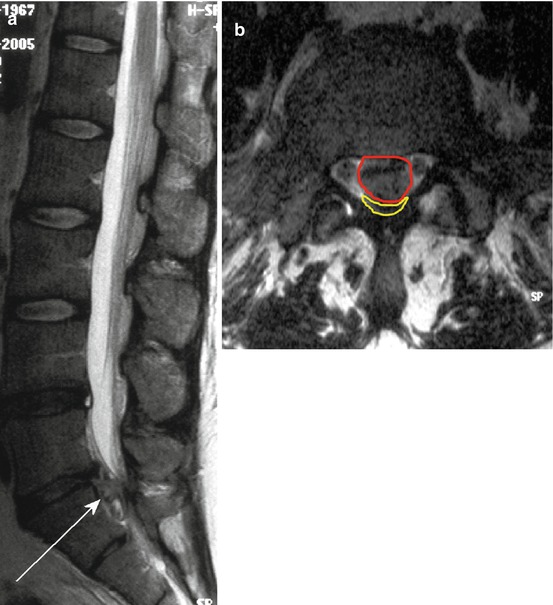
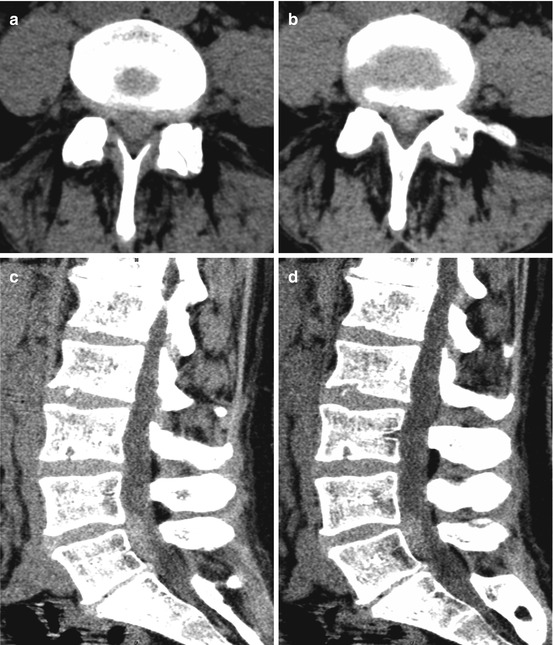

Fig. 10.7
Sag. (a) and ax. (b) T2 WI demonstrating of a medial mass prolapse at the level L5/S1 (a, arrow) in a 38-year-old woman with incomplete cauda equine syndrome; (b) red area show the mass prolapse and yellow small sickle-like formation represent the compressed and dorsal shifted thecal sac enclosing the cauda equine

Fig. 10.8
Axial CT (a, b) and sagittal reconstruction (c, d) showing a hyperdense medial mass prolapse in L4/L5 with suspected caudal sequestration. Despite the extensive prolapsed material the 46-year-old woman showed only a slight sensomotoric L5 syndrome on the right; bladder function as well as deep sacral dermatomas were unremarkable
Comparable to isolated radicular syndromes, differential diagnosis includes inflammatory aetiologies, i.e. polyradiculitis, which may be autoimmune associated (Guillain–Barré syndrome; see also Chap. 13) or caused by viral or bacterial (e.g. borreliosis) infections see Chap. 15). Although lesions of the conus medullaris with affection of the lower sacral segments of the spinal cord could be distinguished from the neurological point of view, in daily clinical practice it is sometimes difficult to separate these two entities. Because of lesions at the S3 to S5 level in the spinal cord, in patients with a conus medullaris syndrome paresis of the legs is absent. Table 10.1 summarizes relevant radicular syndromes.
Table 10.1
Radicular syndromes and neurological deficits
Radicular syndrome | Lowered/extinct deep tendon reflex | Paresis | Sensory deficits |
|---|---|---|---|
C4 | – | Diaphragm muscle | Ventral shoulder |
C5 | (BTR) | M. deltoideus | Lateral shoulder |
M. biceps | Proximal arm | ||
C6 | BTR | M. biceps | Distal arm radial, |
M. brachioradialis | Thumb, index finger | ||
C7 | TTR | M. triceps | Distal arm, dorsal, finger II – IV |
C8 | Trömner reflex | M. abductor minimi | Distal arm, ulnar, finger IV and V |
TTR | Mm. lumbricales | ||
Mm. interossei | |||
L4 | PTR | M. quadriceps | Lateral proximal and medial distal leg |
L5 | TPR | M. extensor halluces longus, M. extensor dig. brevis | Lateral proximal and lateral distal leg; medial foot |
S1 | ATR | M. triceps surae | Dorsolat. proximal and dorsal distal leg, lat. foot |
M. glutaeus max. | |||
Cauda equine syndrome | Level dependency of cauda compression (PTR, TPR, ATR) | Bilateral possible asymmetric flaccid paresis (level) | Sensation deficits in the legs, “Breeches anaesthesia”, bladder and bowel disfunction |
Gait disturbance up to immobility | |||
Conus medullaris syndrome | – | Paresis of the leg absent | “Breeches anaesthesia”, bladder and bowel dysfunction |
10.2.2 Imaging: General Considerations
10.2.2.1 MRI
For spinal MRI sagittal and axial planes are mandatory. Sagittal T1-w TSE (turbo spin echo) sequences best depict the bone marrow and the cortical rims, especially regarding the endplates. The epidural fat should be considered as an anatomical landmark (spinal canal and intervertebral foramen). Anatomical details, e.g. in spondylolysis and other spine diseases, are also well detectable. One problem can be higher field strengths (3 T), because fat is less bright due to longer T1-relaxation times. However, T1-w FLAIR (fluid attenuated inversion recovery) sequence may resolve this problem.
Sagittal T2-w TSE sequences exhibit the best depiction of disc degeneration because of signal decrease of hyperintense nucleus pulposus (see above), best depiction of disc herniation and best depiction of synovial cysts and widening of the facet joints with intraarticular hyperintense signal changes of the joint cavity.
Sagittal T1-w contrast-enhanced sequences may yield inflammatory reaction of adjacent soft tissue caused by degenerative processes, e.g. posterior longitudinal ligament enhancement in disc herniation, facet joints and endplates. In addition, enlarged epidural space adjacent to disc herniation caused by congestion of epidural veins and reactive fibrotic reaction shows enhancement (Fig. 10.9).
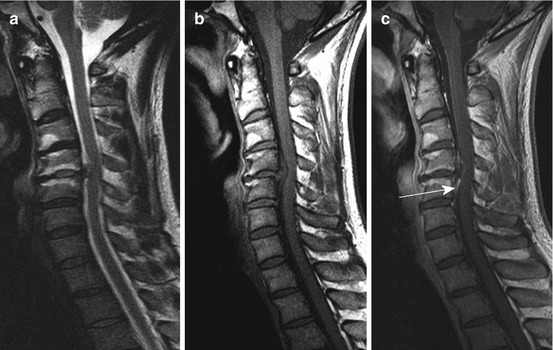

Fig. 10.9
Medial disc herniation at the level C4/5 with exhaustion of the ventral and dorsal subarachnoid space and moderate spinal cord compression. Sag. T2 WI (a) without intramedullary signal conversion; (b, c) sag. T1 WI pc (c) disclosing epidural enhancement near the disc prolapse and partially tended epidural plexus (arrow). Vertebral endplate degeneration at C 4/5 Modic type I, at level C3/4 Modic type II
However, in suspected inflammatory diseases autoimmune associated or caused by bacterial infections (e.g. spondylitis, spondylodiscitis; Figs. 10.2, 10.3 and 10.4 and Chap. 13), T1 WI post contrast are also mandatory. The sagittal “Short-Tau Inversion Recovery” (STIR) sequence is sensitive to increased water content of structures, because of, for example, inflammatory processes showing hyperintense signal changes. Edema in the bone marrow and inflammatory reactions of vertebral structures caused by rheumatic and other autoimmune mediated diseases are especially detectable.
Axial planes on T1-w and T2-w sequences are helpful in classifying disc herniation. Images should depict the herniation completely and small stacks covering only the centre of the disc should be avoided. Delineation of the lateral recessus, the facet joint and the adjacent soft tissue, e.g. ligamentum flavum and synovial structures, is well feasible using axial images.
Coronal planes are optional and should be performed as STIR or T2-w SPIR, e.g. for assessment of paravertebral structures in inflammatory (abscess) and neoplastic diseases (lymphoma). In particular, the psoas muscle is of interest in bacterial infections. Further indications may be sacroiliitis, scoliosis and extraforaminal neurofibroma. The latter sometimes resemble a difficult differential diagnosis in regard to extraforaminal disc herniations, especially when post-contrast images are not performed.
Acquisition of dynamic imaging of the spine during flexion–extension may helpful in assessing instability with pathological movement of the vertebra (Fig. 10.10) and in assessing – with axial loading – load-dependent nerve compression. CT distinguishes soft tissue from bony elements and gas and is especially helpful when analysing bony structures.
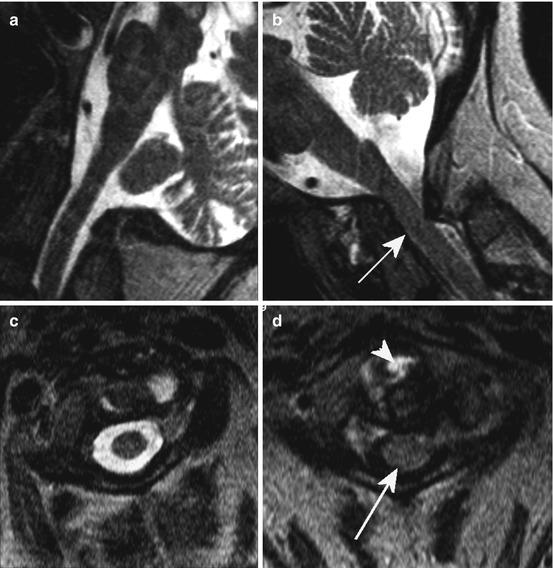

Fig. 10.10
Dynamic imaging of the spine. Pathological mobility at the level C1/2 in a woman suffering from rheumatoid arthritis with severe narrowing of the spinal canal (arrow) in anteflexion on T2 WI (b, d; a, c: retroflexion/extension); arrowhead: inflammatory partially hyperintense reactions around the dens axis
10.2.3 Intervertebral Disc Degeneration
In childhood the gelatinous matrix of the nucleus pulposus show high signals on T2 WI caused by hydration. In adulthood there is a decrease in water-binding capacity and T2 WI shows lowered signal intensity of the nucleus pulposus with increasing collagen content and indistinct boundaries to the anulus fibrosus. Especially in the lower lumbar spine, the discs are often comparatively hypointense on T2 WI reflecting decreasing disc hydration and consecutive smaller intervertebral space because of “flat” discs (Figs. 10.5 and 10.6). However, decreasing signal intensity on T2 WI, dehydration, increasing collagen content and lowered disc height are normal features in aging (see also Chap. 2).
Disc degeneration and thinned disc space induce osteophytosis. Osteophytes originate near the discovertebral junctions or the facet joints caused by traction on Sharpey fibres. The osteophytes extend first in a horizontal and then in a vertical direction.
Advanced disc degeneration may also disclose gas inclusions in the intervertebral disc space (vacuum phenomena; Fig. 10.22).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








