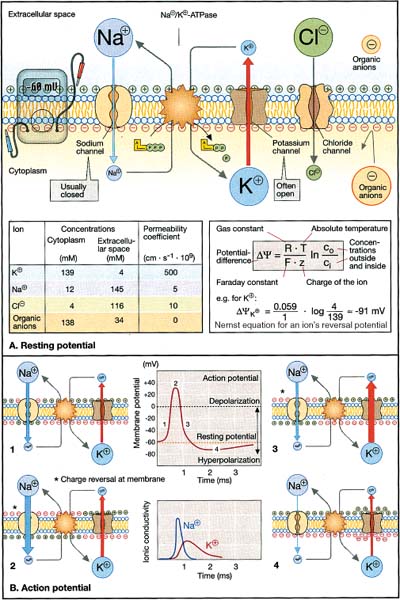
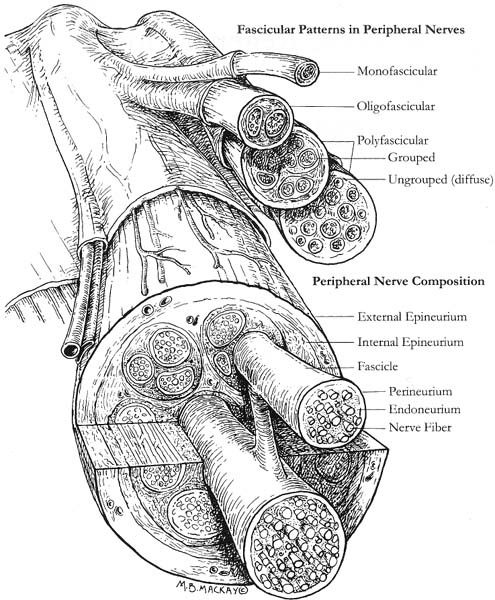
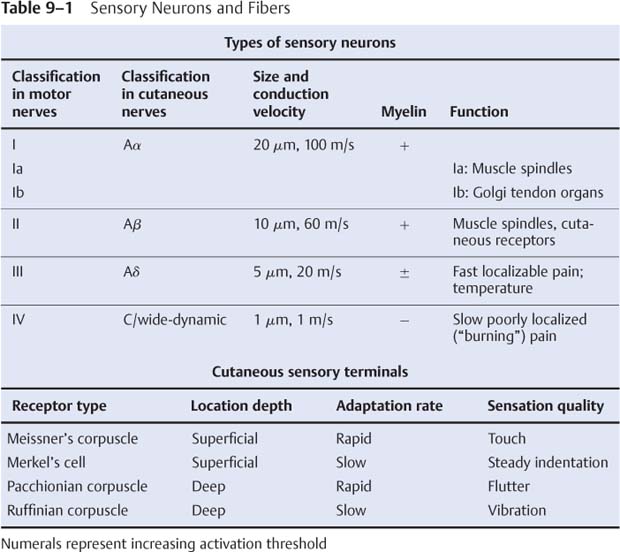
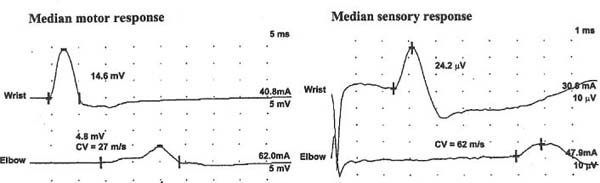
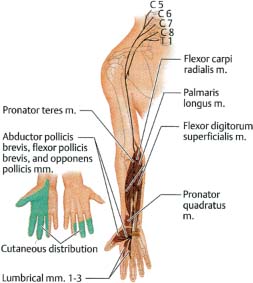
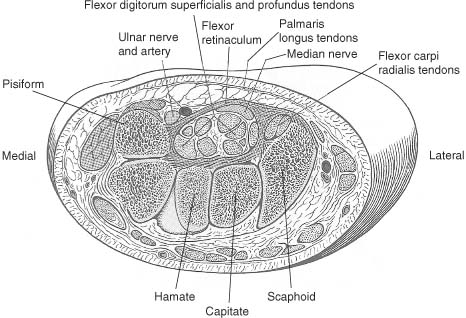
9 Note: Significant diseases are indicated in bold and syndromes in italics. 1. Action potential conduction (Fig. 9–1) 2. Peripheral nerve organization (Fig. 9–2) Figure 9–1 Action potential conduction. (From Koolman J, Rohm KH. Color Atlas of Biochemistry. Stuttgart, Germany: Georg Thieme; 1996:219. Reprinted by permission.) Figure 9–2 Peripheral nerve organization. (From Midha R, MacKay M. Principles of nerve regeneration and surgical repair. Semin Neurosurg 2001, 12:82, Fig. 1. Reprinted by permission.) 3. Peripheral motor axon classification (see p. 231) 4. Sensory receptors and peripheral sensory axon classification (Table 9–1) 1. Electromyography (EMG): see p. 232 2. Nerve conduction studies a. motor nerve conduction studies (Fig. 9–3) i. measures the muscle response to stimulation of a motor nerve (i.e., the compound muscle action potential [CMAP]); CMAP amplitude, distal latency, and conduction velocity can be used to assess the motor nerve integrity after stimulation of both distal and proximal nerve sites, and these measures should be compared against a reference range developed within the testing laboratory ii. usually done with the common peroneal and posterior tibial nerves in the lower extremity, and with the medial and ulnar nerves in the upper extremity b. sensory nerve conduction studies i. performed by stimulating a pure sensory nerve and measuring the amplitude and distal latency of the nerve potential (sensory nerve action potential [SNAP]); usually done with the sural nerve behind the lateral malleolus for the lower extremity, and with the median and ulnar nerves for the upper extremity (1) tests only the integrity of the sensory nerve distal to the ganglion; evaluation of the nerve segment proximal to the ganglion requires somatosensory evoked potentials (SSEPs; see p. 108) (2) detects only dysfunction of large sensory fibers, not small fibers Figure 9–3 Motor nerve conduction study demonstrating partial motor conduction block. The median nerve was stimulated supra-maximally at the wrist and elbow and the motor response was recorded from the abductor pollicis brevis muscle. There is a 67% reduction in CMAD comparing distal to proximal stimulation sites. The antidronic median sensory nerve action potential recorded from the index fingers with ring electrodes was normal note differences in voltage. (From Nagale SV, Bosch EP. Multifocal motor neuropathy with conduction block. Semin Neurol 2003, 23:327, Fig. 1. Reprinted by permission.) ii. measurements of SNAPs usually requires averaging because of their small amplitude c. nerve conduction tests involving the spinal cord i. F waves: an internally-generated anterograde potential from the motoneuron soma caused by retrograde conduction of an action potential that is generated by distal, supramaximal axonal stimulation; the F waves are usually observed as a small response with a 20–50-ms delay after the CMAP that was directly activated by the stimulation {M response} (1) useful for testing the integrity of proximal motor nerve segments (2) can be performed on any motor nerve ii. H reflex: specific stimulation of a sensory nerve that causes reflex activation of motoneurons in spinal cord (equivalent to the reflex arc), > which can then be measured as a myographic response with a long-latency (30-ms delay) following the M response (1) technically best performed by stimulating the tibial nerve while measuring gastrocnemius and soleus muscles (S1 innervation) (2) sensitive to sensory fiber dysfunction more so than motor fiber dysfunction (3) useful for testing integrity of proximal S1 nerve segments, therefore is good for distinguishing sciatic nerve injury from S1 radiculopathy d. technical considerations i. limb temperature variations: cold extremities slows the conduction velocities, and increases amplitudes and distal latencies ii. patient age: slower conduction velocities and reduced amplitudes are normal in young children and in the elderly iii. limb position should be kept constant and compared against a reference standard that was developed for that limb position 3. Nerve biopsy a. diagnostic in some conditions with abnormalities of i. axons (e.g., giant axon neuropathy) ii. myelin (e.g., hereditary neuropathy with liability to pressure palsies, anti-myelin-associated glycoprotein [MAG] antibody neuropathies, leukodystrophies) iii. connective tissue and supportive elements (e.g., vasculitis, amyloid, sarcoid, leprosy, tumor infiltration) b. can be supportive, but not diagnostic, of Charcot-Marie-Tooth neuropathies and inflammatory demyelinating polyradiculopathies (Guillain-Barre syndrome, chronic inflammatory demyelinating polyneuropathy [CIDP]) c. procedure: generally involves both nerve and muscle biopsy simultaneously i. sites for nerve biopsy (1) sural nerve, 15 cm above the heel: complicated by sensory loss with paresthesias and allodynia around the lateral malleolus that generally resolves over a period of years; avoid nerve biopsy at the ankle because repeated trauma from shoes causes nonspecific changes in the nerve (2) superficial peroneal nerve, at the lower third of the anterior calf (3) superficial radial nerve: used only when the neuropathy is limited to the upper extremities or when severe, chronic neuropathy exists in the lower extremities that would prevent useful histo-logical analysis of the nerve (4) intermediate cutaneous nerve of the thigh, at the lower third of the anterior thigh: used mostly to confirm proximal diabetic plexopathy (i.e., diabetic amyotrophy/Bruns-Garland syndrome) Figure 9–4 Wallerian degeneration in situ. (A) Light microscopy demonstrating ovoid myelin debris. (B) Electron microscopy demonstrating degeneration axons in cross-section (arrows). (From Tseng CY et al. Histologic analysis of schwann cell migration and peripheral nerve regeneration in the autogenous venous nerve conduit. J Reconstruct Microsurg 2003, 19:338, Fig. 14. From Oliveira EF et al. Correlation between functional index and morphometry to evaluate recovery of the rat sciatic nerve following crush injury. J Reconstruct Microsurg 2001, 17:73, Fig. 5B. Reprinted by permission.) ii. tissue preparation: sections of the nerve should be evaluated by (1) formalin fixation followed by paraffin embedding, which demonstrates the connective tissue and blood vessels (2) frozen section for immunostaining, which demonstrates leukocytes, complement components, and autoantibodies (3) plastic embedding for high-resolution microscopy (4) teased single nerve fibers, which demonstrates demyelination and remyelination, myelin wrinkling, myelin overlap {tomaculae}, and Wallerian degeneration (Fig. 9–4) 1. Anatomy: involves only the motor roots of C1–4; the sensory roots from those levels are distributed through lesser occipital, greater auricular, transverse cervical, and supraclavicular nerves a. innervates the diaphragm (via the phrenic nerves), scalenes, lower trapezius, infrahyoid/strap muscles, and high cervical paravertebral muscles 2. Pathophysiology: relatively resistant to injury caused by distortion of the neck; injury is more commonly caused by local tumor growth 3. Symptoms a. diaphragm weakness: diaphragm hemiparalysis may present only as orthopnea and exertional dyspnea; complete diaphragm paralysis produces severe dyspnea with inability to cough b. infrahyoid/strap muscle paralysis generally does not produce symptoms Figure 9–5 Brachial plexus anatomy. (From Duus P, Topical Diagnosis in Neurology. Stuttgart, Germany: Georg Thieme; 1998:24, Fig. 1.24. Reprinted by permission.) 1. Anatomy: composed of motor and sensory roots from C5–T1; variants may involve contributions from C4 or T2 2. General symptoms: pain in the neck and shoulders with radiation into the distribution of the affected nerves a. supraclavicular lesions: more commonly injured than infraclavicular sites (severe trauma commonly involves all three trunks) i. upper trunk/C5–6 root injury (1) pathophysiology: caused by (a) traumatic depression of the shoulder (e.g., neck–shoulder distraction, weight-bearing compression) (i) traction trauma more commonly affects nerve trunks than roots, in comparison with lower trunk/C8–T1 injury (b) birth trauma: maternal obesity, multiparity, and large baby size are greater risk factors than obstetrical intervention; can occur even with normal vertex head positioning (2) symptoms (a) weakness in shoulder abduction (supraspinatus and deltoid) and external rotation (infraspinatus), elbow flexion (biceps and brachioradialis), and forearm supination; produces an internally rotated and adducted shoulder with extended elbow and pronated hand {waiter’s tip/Duchenne-Erb palsy} (i) winging of the scapula (weakness of the serratus) suggests involvement of the C5–7 nerve roots, from which the long thoracic nerve originates (ii) weakness of scapula elevation (rhomboid) suggests involvement of the dorsal scapular nerve, which originates from the C5 nerve root (b) sensory loss in lateral brachium and antebrachium ii. middle trunk/C7 root injury (1) pathophysiology: usually occurs in conjunction with upper or lower trunk injuries (2) symptoms (specific for the middle trunk/C7 root) (a) weakness in elbow extension, wrist extension, finger extension, and forearm pronation (pronator teres and quadratus); produces a decorticate-like posture (b) sensory loss in the dorsal brachium, antebrachium, and hand iii. lower trunk/C8-T1 root injury (Box 9.1) (1) pathophysiology: commonly caused by traumatic extension of the shoulder (e.g., catching oneself while falling), breech delivery, and apical lung tumors {Pancoast’s tumor} (a) traction trauma more commonly affects nerve roots than trunks, unlike upper trunk/C5–6 injury (2) symptoms (a) weakness in wrist flexion and all hand intrinsic muscles, resulting in a claw-like posture of the hand {Klumpke’s palsy} (b) sensory loss in medial brachium, antebrachium, and hand (c) ipsilateral mild ptosis, miosis, abnormal accommodation, and reduced face and neck sweating {Horner’s syndrome}, when the T1 motor root is injured prior to its fusion into the lower trunk, i.e., before the white ramus (i) Horner’s syndrome may even involve loss of iris pigmentation and a decrease in intraocular pressure (ii) Horner’s syndrome does not involve a real endophthalmos; it just appears to be so because of the upper and lower lid ptosis b. infraclavicular lesions: less commonly injured than supraclavicular sites; generally are injured by penetrating trauma, shoulder dislocations, clavicular fractures, or irradiation (e.g., for breast cancer treatment) Neurological thoracic outlet syndrome—Caused by a fibrous band from the C7 vertebra transverse process to 1st thoracic rib, which compresses C8 and T1 roots; not related to a cervical rib; symptoms include pain in the ulnar forearm, hand weakness, atrophy of the thenar eminence or that involves the whole hand and medial forearm Arterial thoracic outlet syndrome—Caused by compression of the subclavian artery by a cervical rib; symptoms include pain and weakness from ischemia of the hand and forearm Venous thoracic outlet syndrome—Caused by thrombosis of the subclavian vein; symptoms include cyanosis and swelling of the arm ii. medial cord injury—symptoms include weakness in wrist flexion toward the ulnar side, finger flexion and extension, and hand intrinsic muscles including thumb flexion and opposition (similar to that of Klumpke’s palsy), as well as sensory loss along the whole medial side of the upper extremity iii. posterior cord injury—symptoms include (1) weakness in shoulder abduction (deltoid) and adduction (latissimus dorsi and teres major), elbow extension, forearm supination, wrist extension, and finger extension (2) sensory loss that is usually limited to the thumb and deltoid, but it may extend over the entire radial nerve sensory territory (i.e., dorsal web-space between the first and second digits, the dorsal brachium, and dorsal ante-brachium) 4. Diagnostic testing a. neuroimaging: not reliable for lesion localization in the brachial plexus i. plain radiographs can demonstrate vertebral and clavicular fractures ii. CT myelography and MRI can demonstrate nerve root avulsions and traumatic injury to the meninges and spinal cord; CT myelography is superior to conventional myelography, particularly for C5–6 lesions b. nerve conduction study: results must be interpreted carefully because the presence of injury to the plexus will hide root injuries c. EMG: the absence of fibrillation potentials in paraspinal muscles does not rule-out nerve root injury, but their presence does confirm it 5. Treatment a. anastomosis of transected neural elements, although this is generally ineffective for nerve roots or for injuries to lower trunk b. destruction of the dorsal root entry zone for elimination of chronic pain of nerve root injury Figure 9–6 Lumbosacral plexus anatomy. (From Duus P, Topical Diagnosis in Neurology. Stuttgart, Germany: Georg Thieme; 1998:24, Fig. 1.25. Reprinted by permission.) 1. Pathophysiology: Lesions are caused by a. tumor compression or invasion (Box 9.2) b. retroperitoneal mass (hematoma > abscess) c. pregnancy and delivery d. pelvic irradiation e. trauma (rare), which has to be immensely violent due to soft tissue and bony protection 2. Symptoms a. pain that is worsened with hip extension and often with straight-leg raises (as with radiculopathies); pain is most likely to be absent in radiation-induced plexopathies Malignant psoas syndrome—lumbar plexopathy caused by tumor infiltration of the psoas muscle Warm dry foot syndrome—pain and anhidrosis of the entire lower extremity, caused by tumor invasion of the sympathetic plexus i. lumbar plexus lesions: pain is located in the back, pelvis, or anterior thigh ii. sacral plexus lesions: pain is located in the posterior thigh, calf, or foot b. weakness i. lumbar plexus lesions: weakness mostly in hip flexion and knee extension ii. sacral plexus lesions: weakness mostly in ankle movements and hip extension; involvement of the pudendal nerve function or S2–4 nerve roots causes bowel and bladder dysfunction (rare) 3. Diagnostic testing a. neuroimaging: MRI and radionucleotide scanning are useful for localization of tumors; CT is useful for imaging of hematomas b. EMG: evidence of denervation in the gluteus muscles and the muscles innervated by the sciatic nerve indicates a sacral plexopathy rather than a sciatic neuropathy Figure 9–7 Median nerve anatomy. (From Rohkamm R. Color Atlas of Neurology. Stuttgart, Germany: Georg Thieme; 2004:35. Reprinted by permission.) 1. Pathophysiology: Patients with a preexisting neuropathy are at greater risk for compression neuropathies, but there is no evidence that one focal injury to a nerve increases the susceptibility of that nerve to a second focal injury a. histology i. focal compression causes longitudinal sliding of the myelin layers that collect on either side of the site of compression {tomaculae}; accumulations of excess myelin layers may impair axoplasmic transport ii. Wallerian degeneration order of progression of historical changes (1) Schwann cell retraction and myelin breakdown begin proximal to the injury site; accumulation of mitochondria and transport vesicles occur at the nodes of Ranvier along the length of the axon (2) disintegration of the smooth endoplasmic reticulum in the neuronal soma (3) breakdown of microtubule and intermediate filament structures (4) macrophage invasion of the nerve: a late response, therefore Wallerian degeneration is not a primary inflammatory reaction (5) persistence of Schwann cell tubes and their basal lamina {bands of Bungner} 2. Specific compression neuropathies of the upper extremity a. median nerve compression syndromes (Fig. 9–7) i. carpal tunnel syndrome (1) pathophysiology: compression occurs in the carpal tunnel of the wrist (Fig. 9–8), which is formed by the transverse carpal ligament superiorly and carpal bones inferiorly (a) risk factors include repetitive uses of the hands (50%), obesity, pregnancy, endocrinopathy (i.e., hypothyroidism, acromegaly), rheumatoid arthritis, osteoarthritis, and previous wrist fractures (2) symptoms Figure 9–8 The carpal tunnel. (From Platzere W. Atlas of Topographical Anatomy. Stuttgart, Germany: Georg Thieme; 1985:145, Fig. 153. Reprinted by permission.) (b) paresthesias of hand and fingers, particularly while sleeping or with sustained hand positions (Box 9.3) (i) only 50% of patients will localize the paresthesias within the distribution of the distal median nerve; often the paresthesias will involve all fingers (ii) may be elicited by tapping on carpal tunnel {Tinel’s sign} or by prolonged wrist flexion {Phalen’s sign}, which are insensitive and nonspecific means of inducing paresthesias for any focal nerve injury Unlike carpal tunnel syndrome, C6 radiculopathy has radiating neck pain, loss of sensation in thenar eminence, and weakness in the forearm, wrist flexion, and pronation (c) sensory loss, usually limited to part of the distal median nerve sensory territory; sensory loss should not involve the thenar eminence, which is supplied by a branch of the median nerve that leaves above the carpal tunnel (d) weakness of thumb abduction (abductor pollicis brevis) and opposition (opponens pollicis) causing grip weakness; weakness of flexor pollicis brevis (partly ulnar nerve inner-vated) and 1st–2nd lumbricals are generally asymptomatic (Fig. 9–9) (i) thenar atrophy may develop rapidly in the elderly (3) diagnostic testing (a) nerve conduction study: useful in prognostication because it identifies demyelination (mild injury) or axonal injury (severe injury) (i) often detects abnormalities in asymptomatic individuals, therefore is overly sensitive (b) EMG: may demonstrate the presence of denervation potentials in cases of severe injury (c) serology for comorbid conditions: routinely evaluate for hypothyroidism, diabetes, and pregnancy (4) treatment (a) medical treatment: attempt for 4–6 weeks in patients with mild symptoms; high likelihood of failure in patients who have continuous symptoms or with duration of symptoms > 10 months (i) avoidance of repetitive wrist motions; immobilization with neutral wrist splint (ii) NSAIDS; diuretics if swelling is involved; local glucocorticoid injection (b) surgical treatment: transverse carpal ligament release, either by open procedure or endoscopic approach, in patients with refractory symptoms or in cases with atrophy ii. anterior interosseous syndrome (1)
Diseases of the Nerves
I. Nerve Physiology
II. Diagnostic Testing
III. Plexopathies
A. Cervical Plexus
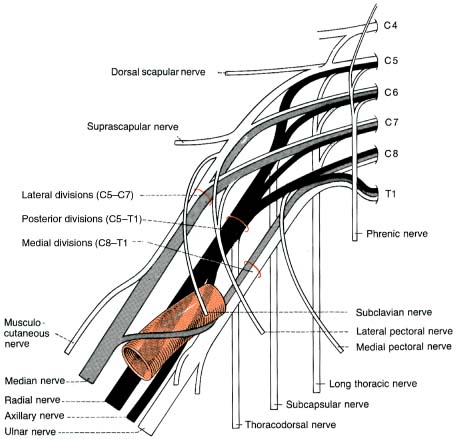
B. Brachial Plexus (Fig. 9–5)
Box 9.1
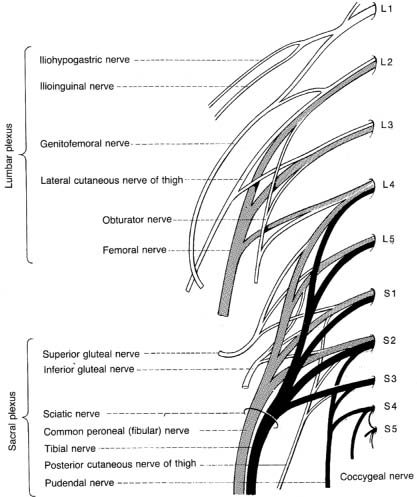
C. Lumbosacral Plexus (L1–S2) (Fig. 9–6)
Box 9.2
IV. Traumatic and Compression Neuropathies (“Entrapment Neuropathies”)
Box 9.3
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree