19
19 
Do Patients with Intracranial Pressure Monitors Need Prophylactic Antibiotics?
BRIEF ANSWER
The literature contains conflicting evidence about the benefits and risks of prophylactic antibiotics for preventing infection of intracranial pressure (ICP) monitors. Several studies supporting prophylactic antibiotic use also demonstrate increased incidence of infection due to unusual or resistant microorganisms. The currently available evidence supports a recommendation (level III) that patients with ICP monitors do not need prophylactic antibiotics other than a single dose of cefazolin 30 minutes prior to the start of the procedure.
Background
The modern era of ICP monitoring began with the report of the use of intraventricular catheters by Guillaume and Janny in 1951.1 Despite subsequent advances in technology, the ventriculostomy catheter remains the ” gold standard“ among ICP monitoring devices. Its widespread use is often considered to be a factor in the improved outcome of neurotrauma patients when compared with earlier outcomes (despite lack of class I evidence to support this assumption).2
The most common complications of ventriculostomy catheters are hemorrhage, overdrainage, and infection.3 The first two may be minimized by meticulous surgical technique, by careful regulation of the amount of cerebrospinal fluid (CSF) drained, and by monitoring of the patient’s clinical condition while ventricular drainage is being used.
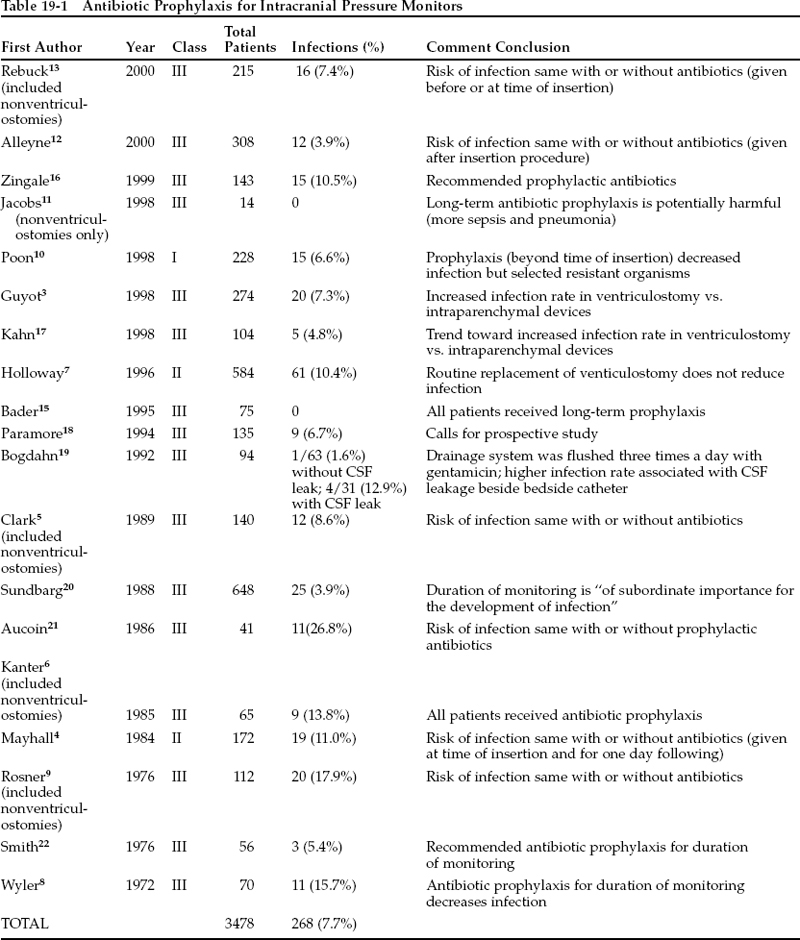
ICP monitor infection has been the subject of investigation, speculation, and controversy for over 30 years. The following factors have been thought to affect the risk of infection: experience and technique of the surgeon; setting of procedure; type of monitor placed; duration of monitoring; patient’s underlying pathology; leakage or flushing of the drainage system; use of steroids; other concurrent infection; and use of prophylactic antibiotics. The infection rates of ICP monitors in selected publications range from zero to 26.8%, with an overall rate of ~7.7% (Table 19-1).
The vast majority of published studies examining risk factors for infection of ICP monitors have been retrospective (class III or, at best, class II). Caution must be used in interpreting such data. Early series showed increased infection in devices that had been in place for a long time, leading some authors to suggest that ventriculostomies should be routinely changed at certain intervals to reduce the chance of infection (class II and class III data).4,5 At least one early study, however, did not support duration of use as a significant risk factor.6
Pearl
Although early series suggested that ventriculostomies should be routinely changed at certain intervals to reduce the chance of infection, subsequent studies have suggested that such a practice may not be necessary.
Later, a well-designed class II study failed to support the hypothesis that the risk:benefit ratio of routine catheter replacement was favorable.7
As used in this chapter, ” prophylactic“ use of antibiotics refers to intravenous administration at the time of insertion of an ICP monitor, with continued scheduled dosing until the device has been removed. Only one class I study examining this question has been published to date. The remainder of the studies report class II or class III data.
Literature Review
Table 19-1 summarizes selected studies pertinent to the question of prophylactic use of antibiotics in patients with ICP monitors. The classes of evidence provided by these studies may be summarized as follows: 16 studies report class III data, two studies describe class II evidence, and one study contains class I data.
In 1972 Wyler and Kelly8 reported a series of 102 ventriculostomies in 70 patients. In this class III review, prophylactic ampicillin reduced the infection rate from 27 to 9%. Three of the four infections in the prophylactic group were due to coagulase-positive Staphylococcusspp., whereas the fourth was due to Serratiasp. The authors concluded that prophylactic antibiotics should be used, and they recommended a more aggressive regimen of either intravenous methicillin or oral cloxacillin to provide better coverage against the gram-positive cocci that caused most of their infections; these organisms were usually coagulase-positive in patients who developed infections despite antibiotic prophylaxis.
Rosner and Becker9 performed another retrospective, multivariate analysis of complications of ICP monitors. They identified monitoring duration but not antibiotic prophylaxis as a significant risk factor for infection in this class III study.
Mayhall et al4 published a prospective series of ventriculostomies that was not randomized with respect to antibiotic use. This class II study reported on two groups: a group receiving no antibiotic prophylaxis, and a group receiving no more than four doses of nafcillin within the first 24 hours of the procedure. The overall infection rate was 11%: 12.9% in the prophylactic group and 6.1% in the group receiving no antibiotics. However, the difference between the groups was not significant, and the authors did not identify antibiotic use as a significant determinant of ventriculostomy infection.
Poon et al10 have reported the only class I study specifically designed to examine antibiotic prophylaxis in ventriculostomy. This study from the Prince of Wales Hospital in Hong Kong randomized ventriculostomy patients to one of two groups: those receiving only perioperative Unasyn (ampicillin/sulbactam) and those receiving Unasyn and aztreonam for the duration of monitoring with a ventriculostomy catheter. Prolonged antibiotic use reduced the CSF infection rate from 11 to 3% (p .01). The infections in the prophylactic group were caused by methicillin-resistant Staphylococcus aureusand Candida albicans, as opposed to Staphylococcusspp., Bacillussp., Escherichia coli, Klebsiellasp., Acinetobactersp., Aeromonas hydrophilia, and Xanthomonas maltophiliain the perioperative group. This finding is similar to the results of Wyler and Kelly8 in that use of prophylactic antibiotics seems to select for resistant or opportunistic organisms. The authors also reported a lower incidence of extracranial infections in the prophylaxis group, but such results have been challenged by others.11
Pearl