13
13 
Does Raising Cerebral Perfusion Pressure Help Head-Injured Patients?
BRIEF ANSWER
Published recommendations for management of cerebral perfusion pressure (CPP) after traumatic brain injury (TBI) range from high values (>70–80 mmHg) intended to improve perfusion of the injured brain to reduced levels (50 mmHg) to minimize edema formation. No randomized trial has compared the results of these different management strategies in terms of neurologic outcome. Class II evidence from published clinical studies suggests that, based on measures of global cerebral blood flow (CBF) and global cerebral oxygenation, a CPP of 60 mmHg provides an adequate perfusion pressure for the majority of adult TBI patients. A single-institution randomized clinical trial (class I evidence) found that maintaining a CPP higher than that required to adequately perfuse the brain reduced the incidence of secondary ischemic insults associated with hypotension, but this management protocol did not result in superior long-term neurologic outcomes compared with simply treating hypotension promptly when it occurred. Moreover, this practice was associated with a fivefold higher risk of developing adult respiratory distress syndrome (ARDS).
Background
The traditional approach to the management of blood pressure and intracranial pressure (ICP) after TBI has been to direct all therapies at the ICP via a stair-step approach. Therapies were progressively added or subtracted as needed based on the response of the ICP, with the primary goal being control of ICP. Although avoidance of hypotension has been recognized as an important principle, the treatment of systemic hypertension, when present, was often one of the treatment modalities used to lower ICP. The outcome standard that is usually cited for this traditional treatment strategy is that from the Traumatic Coma Data Bank (TCDB) series that was published in 1991 (class II data).1 Recently, several groups have advocated different overall strategies for the management of TBI, and each group has claimed improved neurologic outcome compared with the standard set by the TCDB series.
Literature Review
Cerebral Perfusion Pressure Management
Rosner et al2 have advocated the management strategy that is widely known as “CPP management.” This approach is based on the physiologic concept of the “vasodilatory cascade.” According to this hypothesis, a reduction in CPP stimulates the cerebral vessels to dilate in an attempt to maintain CBF. The increase in cerebral blood volume that accompanies the vasodilation further reduces CPP by increasing ICP. This process sets up a cycle that leads to a progressively falling CPP. Based on observations that an increase in blood pressure will break the cycle and reduce ICP, Rosner et al emphasized maintaining CPP by raising mean arterial pressure (MAP) rather than by lowering ICP. Although it was never supported by a randomized trial, this approach became widely accepted. There was felt to be sufficient value in this practice that it was even included in the 1995 and 2000 versions of the Brain Trauma Foundation’s head injury Guidelinesas an option.3 Several multicenter clinical trials of neuroprotective strategies have incorporated this approach to CPP management in their standard treatment regimen.
Lund Therapy
Another approach, called the “Lund therapy,” emphasizes reduction in microvascular pressures to minimize formation of cerebral edema. This treatment strategy focuses on maintaining cerebral perfusion by treating the ICP side of the CPP equation (CPP MAP – ICP). The goals of this approach are to preserve a normal colloid osmotic pressure, to reduce capillary hydrostatic pressures by reducing systemic blood pressures, and to reduce cerebral blood volume by vasoconstricting precapillary resistance vessels. Treatments that would promote transcapillary filtration of fluid, including targeting a high CPP, are avoided. Mortality has been reported to be 8% in a series of 53 patients managed by this approach, with 79% of patients described as achieving a Glasgow Outcome Scale score of good recovery or moderate disability by 6 months postinjury (class II data).4
Individualizing Treatment
Another approach has been to try to match the treatment to the underlying pathophysiology. This approach emphasizes that TBI is heterogeneous and that each individual patient has a predominant pathophysiologic pattern. In addition, it recognizes that the pathophysiology of TBI evolves over time. Treatment that is appropriate during the first few hours after injury may not necessarily be optimal several days after injury.5
Pearl
Different management strategies emphasize different parts of the overall equation that determines CBF: (MAP-ICP)/CVR = CBF where CVR represents cerebral vascular resistence. The CPP management strategy emphasizes increasing the MAP, whereas the Lund therapy emphasizes decreasing the ICP. Individualization of treatment emphasizes normalizing all parameters. The common goal is to improve perfusion of the injured brain.
What Is the Minimum Adequate Cerebral Perfusion Pressure After Traumatic Brain Injury?
The definition of what constitutes an adequate CPP varies with the management approach. Advocates of the Lund therapy consider the optimal CPP to be that which is sufficient for adequate perfusion of the brain. This group argues that a higher CPP does not improve cerebral perfusion and serves only to increase edema in the injured brain. In contrast, advocates of the CPP management approach argue that CPP should be kept above the lower limit of autoregulation. Below this level, the cerebral vasodilation that is induced as the brain compensates for a lower CPP can contribute to intracranial hypertension as long as pressure autoregulation remains intact.
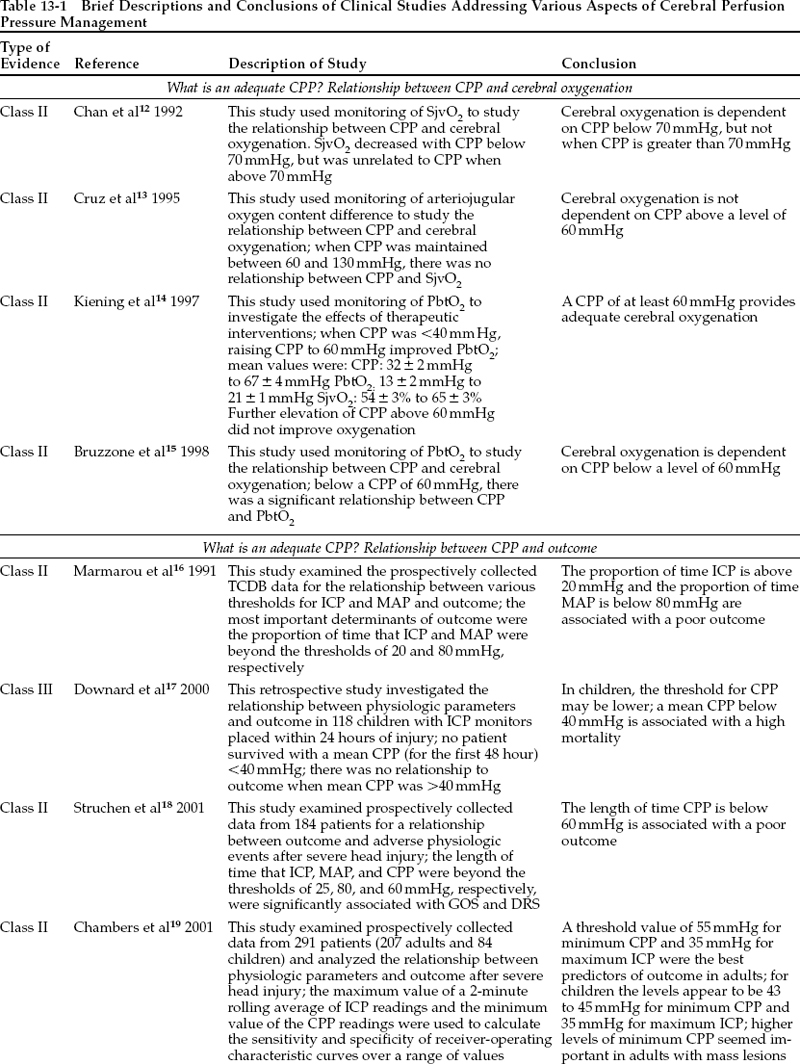
To investigate the minimum safe threshold for CPP after TBI, many prospective clinical studies have examined the relationship between CPP and CBF or between CPP and a measure of cerebral oxygenation, such as jugular venous oxygen saturation (SjvO2) or, more recently, partial pressure of oxygen in brain tissue. In these studies, an increase in CBF or in cerebral oxygenation as CPP increases is interpreted as indicating that further elevation of CPP would improve cerebral perfusion. Lack of change of CBF or of cerebral oxygenation in response to increases in CPP is interpreted as indicating that CBF is above the lower limit of autoregulation and is adequate for metabolic needs. As shown in Table 13-1, interpretation of the data in this manner yields a critical CPP threshold of 60 to 70 mmHg. One caution in accepting this result is that regional ischemia can occur despite seemingly adequate global oxygenation,and focal areas of the brain may be underperfused even at normal CPP levels (class II data).6 In these circumstances, elevation of CPP beyond 60 to 70 mmHg can improve regional cerebral oxygenation.
Other prospective clinical studies have examined the relationship between different thresholds for CPP and outcome from TBI. These studies are limited in that a cause-and-effect relationship cannot be assumed even though an association with outcome can be demonstrated. Nevertheless, as shown in Table 13-1, these studies support the same general concept of a CPP threshold of 60 mmHg in adults.
The available information suggests that it is probably most correct to conclude that, after TBI, an adequate CPP is necessary but not sufficient to guarantee that CBF is adequate. Measurements of global CBF and global cerebral oxygenation in the available clinical studies suggest that a CPP of 60 mmHg provides an adequate perfusion pressure for the majority of adult TBI patients. It must be emphasized, however, that regional ischemia may occur despite adequate global cerebral oxygenation.
Pearl
A CPP of 60 mmHg seems to provide an adequate perfusion pressure for the majority of adult TBI patients. It must be emphasized, however, that regional ischemia may occur despite adequate global cerebral oxygenation.
Does Increasing Cerebral Perfusion Pressure Above 60 mmHg Have a Beneficial Therapeutic Effect?
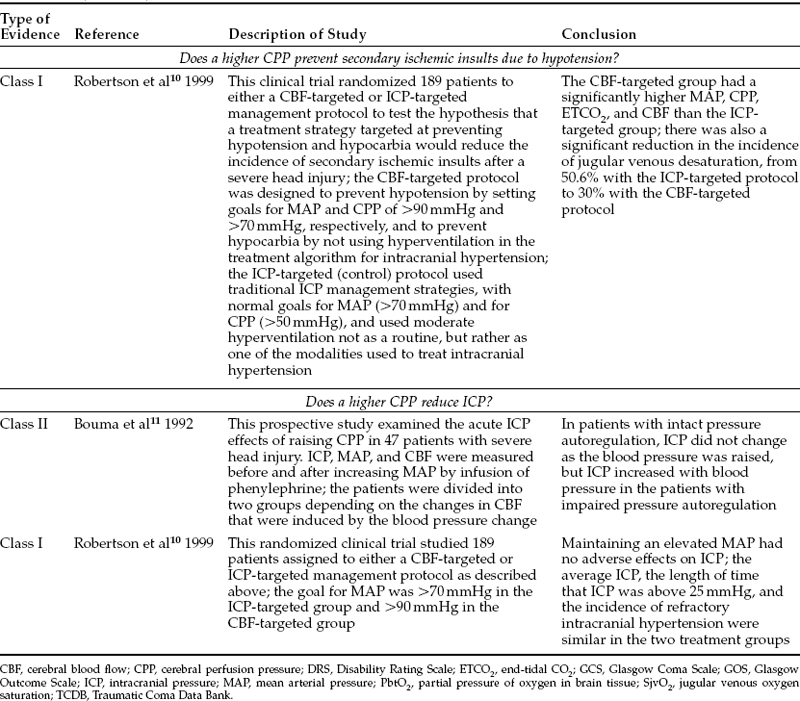
There exists general agreement that CPP should be kept at a level that provides adequate perfusion of the injured brain; that is, at least 60 mmHg. The practice of raising CPP to even higher levels could have both beneficial effects and detrimental consequences. The risk:benefit ratio of this practice, and therefore its overall effect on outcome, is controversial. No multicenter randomized clinical trials have compared these different management strategies in terms of neurologic outcome. One single-site randomized clinical trial and numerous prospective clinical studies do address potential benefits of elevating CPP, including fewer secondary ischemic insults and lower ICP. In addition,these studies give some information on outcome effect. They are summarized in Tables 13-1 and 13-2.
Does a Higher Cerebral Perfusion Pressure Prevent Secondary Ischemic Insults Due to Hypotension?
Several investigators have linked secondary ischemic insults to poor neurologic outcome (class II data).7–9 Thus, one might reasonably expect that a potential benefit of keeping CPP at an elevated level might be a reduction in the incidence of secondary ischemic insults.
One randomized clinical trial has addressed this question directly. Robertson et al10 randomly assigned 189 patients to either a CBF-targeted or an ICP-targeted management protocol to test the hypothesis that a treatment strategy targeted at preventing hypotension and hypocarbia would reduce the incidence of secondary ischemic insults after severe TBI (class I data). The CBF-targeted protocol was designed to prevent hypotension by setting goals for MAP and CPP at >90 mmHg and >70 mmHg, respectively, and to prevent hypocarbia by eliminating hyperventilation from the treatment algorithm for intracranial hypertension. The ICP-targeted protocol consisted of a traditional ICP management protocol with goals for MAP and CPP of >70 mmHg and >50 mmHg, respectively, and allowed moderate hyperventilation not as a routine, but rather as one of the modalities used to treat persistent intracranial hypertension. Because of the differences in the management protocols, the CBF-targeted group had a significantly higher MAP, CPP, end-tidal CO2, and CBF than the ICP-targeted group. The outcome of the study demonstrated a significant reduction in the incidence of jugular venous oxygen desaturation, from 50.6% in the ICP-targeted group to 30% with the CBF-targeted protocol.10
One criticism of this study has been that, although the difference in CPP between the two treatment groups was statistically significant, the absolute difference in the median values for CPP in the two groups was not large (77 and 73 mmHg in the CBF- and ICP-targeted groups, respectively; p = .004). However, the purpose of the CBF-targeted protocol was to prevent hypotensive episodes, and summary values such as mean or median are very insensitive measures for detecting transient, but still important, differences. The length of time that CPP was <60 mmHg was much longer in the ICP-targeted group: a median of 13 hours, compared with 4 hours in the CBF-targeted group (p = .008). Perhaps even more importantly, the length of time that SjvO2 was low because of hypotension totaled 58.9 hours for the entire ICP-targeted group but only 7.8 hours in the CBF-targeted group. Finally, to achieve the desired difference in MAPs, significant differences in treatment were required; e.g., higher fluid intake and more frequent use of pressors.
Another criticism of the study has been that two physiologic parameters (blood pressure and PaCO2) were varied in the same trial, potentially making it impossible to state conclusively that the reduction in ischemic insults was due to targeting a higher CPP. To put this point in perspective, it is important to remember that avoiding hyperventilation has always been a part of the CPP management strategy as described by Rosner et al.2 Also, the physiologic effects of manipulation of blood pressure and PaCO2 are different, and the end point of jugular venous desaturation can be further categorized according to the underlying cause of the desaturation. The incidence of hypotension-induced jugular venous desaturation was significantly lower in the CBF-targeted group. Nevertheless, it is probably more correct to interpret the data as showing that a management strategy designed to minimize both hypotension and hypocarbia significantly reduced the incidence of secondary ischemic insults.
Does a Higher Cerebral Perfusion Pressure Reduce Intracranial Pressure?
Manipulation of blood pressure in TBI patients has a variable effect on ICP. Both initial and delayed effects must be considered. The initial ICP response to increased MAP depends on the status of pressure autoregulation. Bouma et al11 published the results of 58 tests of autoregulation in 47 patients in whom phenylephrine was used to elevate mean arterial blood pressure (class II data). The CBF response to elevation of MAP was used to divide patients into two groups: those with intact pressure autoregulation and those with defective pressure autoregulation. In the group with intact autoregulation, MAP increased from 93±11 mmHg to 119±11 mmHg, but ICP and CBF did not change significantly. In the group with defective autoregulation, MAP increased from 99±12 mmHg to 126±9 mmHg, ICP increased from 16±7 mmHg to 20±9 mmHg (p <.01), and CBF increased from 35±10 mL/100 g/min to 50±14 mL/100 g/min (not statistically significant).
Raising CPP, however, could theoretically increase cerebral edema, with the long-term effect of actually increasing ICP and prolonging the need for treatment. Evidence to support such a possibility may be found in Rosner et al’s own series. As shown in Table 13-2, although the average CPP in Rosner et al’s patients was higher than that in any other listed series, the average ICP was also 8 to 10 mmHg higher than in the other reports. In addition, the average duration of “acute monitoring” in the Rosner et al series was 17.7 days. If this parameter represents the length of time that ICP monitoring was required,then it far exceeds the median of 4 to 5 days reported in the other studies.
The randomized clinical trial by Robertson et al10 described above also examined the effect of the CBF-targeted protocol on ICP control as a secondary outcome measure. In this study, no significant differences existed between the two treatment groups in duration of ICP monitoring, in any measure of ICP severity (mean ICP, length of time ICP was greater than 25 mmHg, or number of patients who died of refractory intracranial hypertension), or in any measure of ICP treatment.
Does a Higher Cerebral Perfusion Pressure Improve Neurologic Outcome?
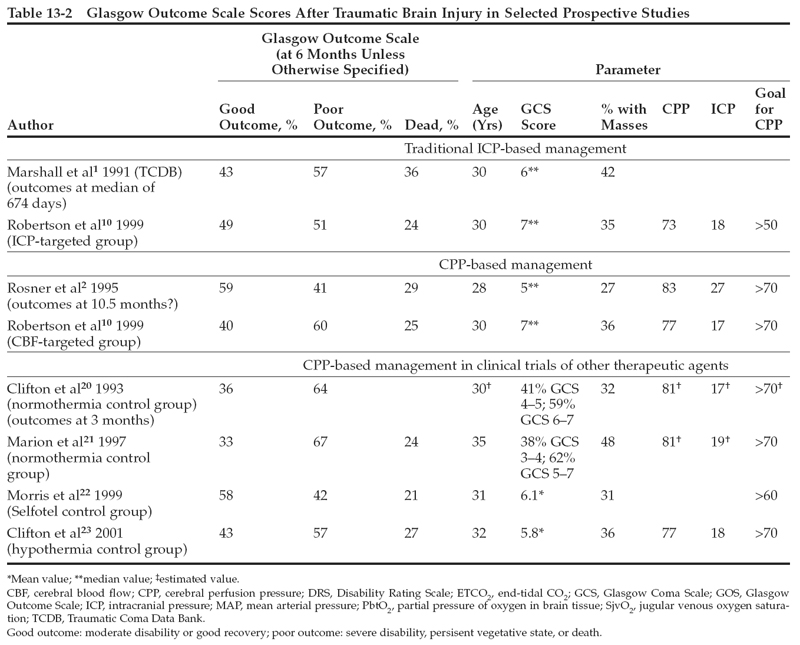
The TCDB study published by Marshall et al1 describes outcomes in a multicenter prospective study of 746 TBI patients with Glasgow Coma Scale scores <9. No prescribed treatment protocol was used in the study, but the investigators all followed traditional ICP treatment strategies. The overall mortality rate at 6 months postinjury was 36.3%. At 6 months, 42.9% of the patients had a favorable outcome (good recovery or moderate disability), and 57.1% of the patients had a poor outcome (severe disability, vegetative state, or death).
Rosner et al2 reported a prospective series of 158 patients managed with a protocol based on his CPP management strategy. The average CPP in this clinical series was 83±14 mmHg (ICP 27±12 mmHg and MAP 109±14 mmHg). Mortality was 29%, and 59% of the patients achieved good recovery or moderate disability by 10.5 months postinjury (class II data).2 These outcomes appear to be improvements over the results of the TCDB series. However, different demographic characteristics in the two groups of patients might also explain the better outcome in Rosner et al’s series, including the lower age (28 vs. 30 years) and the lower incidence of mass lesions (27% vs. 42%) in the Rosner et al series.
Several recent clinical studies provide additional prospectively collected information on outcomes of head-injured patients managed with protocols that include maintaining CPP >70 mmHg. Table 13-2 lists selected parameters from several studies, including the CPP management paper by Rosner et al, the control groups from some recent multicenter clinical trials that followed a CPP management strategy, and the CBF-targeted group from the Robertson study. Although most of these groups of patients share the management goal of keeping CPP >70 mmHg, the studies cannot be directly compared because of differences in patient demographics, in entry/exclusion criteria, and in other aspects of treatment. Despite these potentially confounding factors, however, none of these prospectively collected clinical series, either alone or collectively, demonstrates convincingly a beneficial effect on outcome from maintaining CPP >70 mmHg.
The randomized trial by Robertson et al10 examined long-term neurologic outcome as a secondary outcome measure. The results, which are also summarized in Table 13-2, did not show a significant improvement in outcome in the CBF-targeted group. The sample size for this study was powered to enable detection of a 20% improvement in favorable outcome, which is probably too large an effect to expect from the difference in management practice that was studied. Nevertheless, the investigators could not find even a suggestion that outcome was improved by the CBF-targeted management strategy. When potential complications of maintaining an elevated CPP were explored, the investigators found a fivefold increase in the incidence of ARDS. This complication may have offset any potential beneficial effects.
Because the only randomized trial that has compared the consequences of targeting different levels of CPP failed to demonstrate a long-term benefit and, in fact, demonstrated a clearly detrimental effect (increased incidence of ARDS) associated with a CPP target of >70 mmHg,there exists no compelling reason to raise CPP in all patients beyond that required to adequately perfuse the brain.10 It seems likely that a CPP of 60 mmHg provides adequate perfusion for most cases. Higher levels of CPP should probably be reserved for those TBI patients who demonstrate a specific indication for induced hypertension, for example, regional cerebral ischemia.
Pearl