MAJOR DEPRESSIVE DISORDER
MDD typically presents with a complex set of overlapping symptoms in varying degrees of severity. These symptoms can be classified as psychological, behavioral, and physical. According to the Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV), two of the essential features of MDD are depressed mood and loss of interest or pleasure in nearly all activities for a period of at least 2 weeks. Emotional, psychological, and cognitive symptoms of MDD may also include anxiety, irritability, reduced concentration and motivation, feelings of hopelessness and helplessness, excessive guilt, thoughts of suicide, hypersensitivity to criticism, low self-esteem and feelings of worthlessness, and indecisiveness. Common behavioral symptoms include psychomotor retardation or agitation, crying spells, anger attacks and interpersonal confrontation, social withdrawal, reduced productivity, compulsive or ritualistic behaviors, substance abuse, and self-injury. Physical symptoms of MDD comprise a third and equally important set of manifestations. These include sleep disturbances, changes in appetite and weight, fatigue, overall aches and pains, gastrointestinal disturbances, backaches and headaches, and sexual dysfunction. Some of these symptoms, of course, overlap with those of a variety of medical conditions, complicating the differential diagnosis. However, for many patients with MDD, these physical symptoms are an important part of their initial presentation. Given the comorbidity of MDD and various medical conditions, a diagnosis of a coexisting medical condition does not exclude a diagnosis of MDD. The DSM-IV diagnostic criteria for MDD, in addition to depressed mood, are captured by the mnemonic SIGECAPS (S for Sleep disturbances; I for Interest, diminished; G for Guilt, excessive or feelings of worthlessness; E for Energy, diminished; C for Concentration, diminished or indecisiveness; A for Appetite disturbances; P for Psychomotor retardation or agitation; and S for Suicidal thoughts or behaviors). MDD is heterogeneous clinically and likely heterogeneous in etiology.
The lifetime risk of MDD ranges from 7% to 12% in men and from 20% to 25% in women. Medical and psychiatric comorbidities are very common in MDD. It has been estimated that only 20% to 25% of the patients with MDD in the community receive adequate treatment. For patients who meet the
DSM-IV criteria for MDD, it can be expected that approximately 30% to 40% will achieve remission with a single adequate trial (i.e., adequate dose for at least 6 weeks) of any effective antidepressant. Of the remainder, the majority will show some improvement, but 15% to 30% will not improve. The most common reasons for failure of drug treatment are
inadequate drug dosage, intolerance to the pharmacological treatment, and inadequate duration of drug trial. For those who respond inadequately to initial treatment, there are dozens of alternatives when one considers the various permutations of switching
agents, as well as augmenting and combining treatments. Most patients who fail adequate trials of drug therapy for moderate to severe depression could respond to electroconvulsive therapy (ECT) if the treatment were available and acceptable to the patient. However, for most of these patients, a continuation and maintenance treatment would still be required, a need that usually calls for continued efforts to find an effective pharmacologic regimen. Recent studies have suggested that some forms of short-term psychotherapy [cognitive-behavioral therapy (CBT) and interpersonal therapy (IPT)] may be as effective as pharmacotherapy in MDD and that the combination of an antidepressant with cognitive therapy can be more efficacious than either treatment alone. Some researchers have argued that the more serious the depression, the clearer the advantage of drug therapy over psychotherapies in terms of efficacy. In fact, there are no published, adequately powered, comparative studies between CBT or IPT and pharmacotherapy in severe and melancholic depression. Nonetheless, many clinicians would argue that given the effectiveness of medications there would need to be compelling evidence before using CBT or IPT as a single initial therapy for melancholic and/or severely ill depressed patients. For residual depressive symptoms that persist despite pharmacotherapy, CBT and its variants may prove to be particularly useful and the improvement in residual symptoms may remove one of the common risk factors (negative cognitions) for recurrence of depression. The combination of pharmacotherapy and IPT has also been shown to reduce the risk of recurrences more effectively than pharmacotherapy alone, at least in a large sample of geriatric depressed patients.
SUBTYPES OF DEPRESSIVE DISORDERS
The DSM-IV criteria for depression are very useful for clinical practice and research but likely include heterogeneous subgroups based on genetic and other risks and on pathophysiology. Attempts to identify more homogenous groups of depressive disorders are currently ongoing. In the meantime, it has been possible to identify groups of patients with specific clinical features that may predict differential treatment responses.
Melancholic Depression
The DSM-IV uses the term melancholic depression to describe severely depressed patients who are unable to experience pleasure (anhedonia) and who lose normal emotional responsiveness to life experiences. These patients also exhibit early morning awakenings, excessive guilt, reduced appetite and weight loss, and psychomotor retardation and agitation. In this population, serotonin-norepinephrine reuptake inhibitors (SNRIs) and those tricyclic antidepressants (TCAs) that act on both the neurotransmitters have shown fairly consistently a modest but significant superiority over selective serotonin reuptake inhibitors (SSRIs).
Anxious Depression
The term anxious depression historically has been used to refer to depressed patients with prominent anxiety symptoms. Many of these patients suffer from comorbid anxiety disorders, whose onset may have either preceded or followed the onset of MDD. Patients with anxious depression are also significantly more likely than patients with nonanxious MDD to be unemployed, to have less education, to be more severely depressed, and to report more melancholic/endogenous features, even after adjustment for severity of depression. Patients with anxious depression have been shown to be less likely to respond to antidepressant treatment than do patients with nonanxious depression, but there is no clear evidence yet of differential antidepressant treatment responsiveness, despite the common belief that certain antidepressants or antidepressant classes may be more anxiolytic than others. However, there is some preliminary evidence that SNRIs may be relatively more efficacious than SSRIs in this subtype. In addition, given the relative modest efficacy of antidepressant monotherapy with these patients, clinicians often augment antidepressants with antianxiety, anticonvulsant, and antispychotic drugs, despite the limited evidence for their efficacy.
Atypical Depression
In the
DSM-IV, atypical depression refers to a subtype of depression characterized by mood reactivity, accompanied by symptoms such as hypersensitivity to rejection or criticism, hypersomnia, hyperphagia (often related to carbohydrate craving), and prominent physical fatigue (leaden paralysis; e.g., feelings of heaviness in arms and legs as if they were full of lead). These patients respond better to monoamine oxidase inhibitors (MAOIs; phenelzine is best studied) than to TCAs, although TCAs are superior to placebo. The only adequately powered study in the literature comparing SSRIs with TCAs and placebo has shown comparable efficacy for TCAs and SSRIs, although both drugs were superior to placebo. One of the theories concerning the superior efficacy of MAOIs over TCAs is that the effect of MAOIs on dopamine neurotransmission may play a key role in this patient population. Given safety and tolerability concerns about MAOIs, some clinicians thereby favor antidepressants with norepinephrine and dopamine mechanisms such as bupropion or antidepressant augmentation with psychostimulants, modafinil, and dopamine D
2 and D
2 receptor agonists such as pramipexole or
ropinirole. Despite the lack of evidence for superiority of SSRIs over TCAs, SSRIs are often used as first-line treatment of atypical depression, and the switch to bupropion or the augmentation with dopaminergic agents is typically pursued only if the patient fails to respond to an SSRI.
Depression with Anger Attacks
Past attempts to classify subtypes of MDD yielded a possible hostile depressive subtype. Recent work indicates that a significant proportion (30% to 40%) of outpatients with MDD are predominantly irritable when depressed and manifest intermittent outbursts of anger or rage, which have been termed anger attacks. Anger attacks emerge abruptly with minimal interpersonal provocation and are associated with a paroxysm of autonomic arousal reminiscent of panic attacks but feature explosive verbal or physical anger, usually directed at close companions or family members. Both anecdotal and systematically ascertained data suggest an important therapeutic role for antidepressants, especially SSRIs, in these patients. In fact, anger attacks cease in the majority of patients with MDD treated with antidepressants. Interestingly, these patients also appear to have decreased central serotonergic activity compared with patients without anger attacks. The depression in irritable patients with anger attacks responds to antidepressants as well as it does in patients without anger attacks.
Secondary Mood Disorders
A large number of medical illnesses and drugs can produce secondary depressive syndromes (
Table 3.1). When the depression is due to a treatable disorder or medication, it may remit with appropriate medical care or discontinuation of the offending drug. If, however, the depression is severe or does not remit after treatment of the medical condition, it is reasonable to initiate antidepressant therapy. For example, certain neurologic disorders (e.g., stroke, Parkinson’s disease, Huntington’s disease) are commonly associated with MDD. Dominant hemisphere stroke patients, in particular, develop MDD with an incidence greater than would be predicted by the degree of disability. Aggressive treatment of the depression may improve the patient’s quality of life and his or her ability to participate in rehabilitation. For patients with depression secondary to an untreatable medical or
neurologic illness, the duration of therapy is undetermined. However, many patients with brain injuries or neurodegenerative disorders (e.g., Alzheimer’s disease) manifest elevated susceptibility to the side effects of psychotropic medications, especially typical antipsychotics, any compounds with anticholinergic activity, and sedative-hypnotics including benzodiazepines. Thus, medications must be prescribed with care.
Grief, Bereavement, and Loss
Following bereavement, loss of a job, or a life event leading to significant loss of self-esteem, individuals may experience symptoms of depression. It is important to distinguish depressive illness from normal grief or sadness. Although normally grieving individuals commonly sleep poorly and have decreased appetite, poor concentration, and other apparent neurovegetative symptoms immediately following the loss, these symptoms improve spontaneously over several weeks’ or months’ time in most cases. If depressive symptoms are particularly severe, persistent, or pervasive; are accompanied by serious suicidal thoughts or behavior; or are protracted beyond what might be reasonably expected for the precipitating stressor, treatment is indicated.
In many cases, the preferred treatment modality is psychotherapy aimed at helping the patient develop adequate coping skills to deal with the loss and associated problems. However, if the depressive symptoms are severe and unremitting, antidepressant therapy should be considered. Indeed, patients are thought to be better able to engage in psychotherapy if severe depressive symptoms are alleviated. Dosages are the same as for the treatment of MDD occurring outside the setting of loss.
Depression with Psychotic Features
MDD accompanied by psychotic symptoms (e.g., delusions or hallucinations) responds poorly to treatment with antidepressants alone. Controlled studies demonstrate that depression with psychotic features is more effectively treated with the combination of an antidepressant and an antipsychotic drug (70% to 80% exhibiting significant improvement) than if treated with either class of drugs alone (30% to 50% response rate). Although some reports describe the efficacy of monotherapy with SSRIs in psychotic depression, the accuracy of diagnosis in these studies has been questioned. ECT is at least as effective as the combined antidepressant -antipsychotic regimen and is the treatment of choice if this combination fails.
The recommended dosage of antipsychotic medication for depression with psychotic features has not been clearly established, but it appears that slightly lower dosages of conventional and second-generation antipsychotic drugs than those used in schizophrenia may be adequate, in addition to full doses of an antidepressant. Individual dosage adjustments are then made as needed. Although exhibiting fewer of the anticholinergic, sedative, and hypotensive side effects of tricyclics, SSRIs may exacerbate the extrapyramidal side effects of both typical and second-generation antipsychotic drugs. Moreover, because of their relatively greater potential to inhibit the hepatic metabolism of drugs metabolized by the P450 2D6 isoenzyme, fluoxetine and paroxetine are more likely than sertraline, citalopram, or escitalopram to produce increases in typical antipsychotic drug levels and thus side effects or toxicity. In contrast to the SSRIs, the anticholinergic effects of TCAs offer some levels of prophylaxis against extrapyramidal symptoms; thus, additional anticholinergics should not initially be prescribed when tricyclics are used in combination with a typical antipsychotic drug. Tricyclics should not generally be combined with low-potency typical antipsychotic drugs (e.g., thioridazine, mesoridazine, or chlorpromazine) because of additive anticholinergic toxicity and postural hypotension. If multiple
anticholinergic medications prove necessary for extrapyramidal symptoms, careful monitoring for anticholinergic toxicity is important. Fixed combinations (e.g., perphenazine/ amitriptyline, fluoxetine/olanzapine) are available to treat depression with psychotic features but limit the flexibility of the clinician to adjust medications individually as needed. Amoxapine, a cyclic antidepressant with metabolites possessing some antipsychotic potential, is used rarely as monotherapy to treat psychotic depression; there are scant supporting data for this approach.
It should be recalled that antipsychotic drugs, particularly the first generation ones, may cause apathy, akinesia, and blunting of affect, which can be confused with depressive symptoms; thus, other target symptoms, such as sleep, guilt, or psychotic symptoms, may be better indicators of improvement when patients are on combined antipsychotic-antidepressant regimens.
Increasingly, the second-generation antipsychotics (e.g., olanzapine, risperidone, quetiapine, ziprasidone, aripiprazole, and paliperidone) have replaced the first generation antipsychotics for use in mood-disordered patients with psychotic features given the relative lack of extrapyramidal adverse effects and the reduced risk for tardive dyskinesia for which mood-disordered patients appear to be more vulnerable. These drugs also appear to offer some antidepressant potential. Whether they might be used alone, without antidepressants, for psychotic depression remains to be demonstrated. A recent study has yielded fairly low response rates to monotherapy treatment with second-generation antipsychotic agents in psychotic depression, suggesting that the old principle of combining antidepressants and antipsychotics in this population may still be valid even in the era of second-generation antipsychotic agents.
Given the serious morbidity and high suicide risk in depression with psychotic features, maintenance treatment is recommended. However, there are few data to guide the decision of whether to continue with combined treatment or either agent alone. For a patient who continues to do well, usual clinical practice involves the gradual (over 3 or 4 months) discontinuation of the antipsychotic drug first, while maintaining the antidepressant treatment for the longer term. In some cases, however, even the gradual discontinuation of the antipsychotic drug may precipitate the emergence of significant symptoms and patients may be kept on the combination therapy for the longer term.
Depression Comorbid with Other Psychiatric Disorders
More than 50% of patients with MDD suffer comorbid psychiatric disorders, in particular, anxiety disorders, substance use disorders, and personality disorders. Relatively higher rates of comorbidity are observed among individuals with observed among individuals with early-onset MDD, suggesting that comorbid conditions may in fact be risk factors for the development of MDD.
Depression with Comorbid Anxiety Disorders
Anxiety disorders commonly accompany MDD and generally respond to antidepressant treatment along with the symptoms of the depressive episode. Because SSRIs and SNRIs are antidepressants with Food and Drug Administration (FDA) approval for certain anxiety disorders, such as obsessive-compulsive disorder (OCD), generalized anxiety disorder, panic disorder, and social anxiety disorder, many clinicians use SSRIs and SNRIs as the first-line treatment of MDD with comorbid anxiety disorders. As in the case of anxious depression, many clinicians also administer adjunctive benzodiazepines, second-generation antipsychotics, or anticonvulsants to target comorbid anxiety disorder symptoms. Adjunctive benzodiazepines offer initial relief of anxiety symptoms prior to onset of antidepressant efficacy. They also may be useful for residual symptoms that do not improve with the antidepressant. Because of its delayed onset of efficacy, the antianxiety
drug buspirone is not used to provide initial relief but may be used to help with comorbid generalized anxiety. It is important to recognize that antidepressants are the essential therapeutic agents in these cases and that full antidepressant doses are needed whether or not a benzodiazepine produces initial improvements in anxiety and insomnia. Combining high-potency benzodiazepines with SSRIs for initiation of antidepressant treatment has been reported to enhance treatment compliance and improve early response but can be followed by tapering off the benzodiazepine as the antidepressant response emerges.
Depression with Comorbid Personality Disorders
Personality disorders often co-occur with MDD. That said, it is treacherous to make a new personality disorder diagnosis during a depressive episode; some studies have shown that patients diagnosed with a personality disorder prior to treatment for their mood disorder no longer met criteria for a personality disorder after treatment. Although initial reports suggested that the presence of comorbid personality disorders, particularly borderline personality disorder, was associated with relatively poorer treatment outcome in MDD, subsequent reports using monotherapy with SSRIs have failed to support this view. SSRIs are often used in patients with borderline personality disorder and other cluster B personality disorders to (a) treat intercurrent MDD; (b) reduce chronic depressive symptoms that do not meet criteria for MDD; (c) modulate anger, hostility, and irritability; (d) diminish impulsivity; and (e) improve other comorbid conditions such as bulimia nervosa or panic disorder. Because personality disorder patients with MDD are often impulsive, angry, and self-destructive, SSRIs also make good first-choice medications because they are less dangerous and lethal in overdose than TCAs or MAOIs. Moreover, in one report, the TCAs amitriptyline and desipramine produced worsening in some such patients— both in self-destructive episodes and in global ratings.
Depression Comorbid with Substance Use Disorders
A substantial proportion of patients with MDD have either current or lifetime history of substance use disorders. In some cases, the substance abuse emerges in the context of already existing MDD, whereas in others, MDD may occur in the context of alcohol abuse and abuse of other central nervous system (CNS) depressants (e.g., barbiturates). In the latter case, often the depressive symptoms are presumed to be due to effects of the alcohol or the other substance of abuse; ideally, therefore, the primary treatment should be detoxification. Given the possibilities of drug interactions with alcohol or barbiturates (including altered pharmacokinetics and possible additive CNS depression), prescription of the older antidepressants to actively drinking alcoholics should be avoided if possible, and the newer antidepressants, safer in overdose, should be preferred. Generally, antidepressants are indicated only if depressive symptoms persist for 4 weeks or more after successful detoxification or if the history indicates that mood disorder may be primary and not secondary to substance abuse. There is some support for the potential of SSRIs to reduce drinking for some patients unrelated to an antidepressant effect and for antidepressant treatment to increase the likelihood of abstinence in depressed alcoholics. There also has been interest in the possibility that antidepressants may help maintain abstinence from cocaine based on early reports with desipramine and later anecdotes involving fluoxetine or other SSRIs, but the evidence for the usefulness of antidepressants in nondepressed cocaine abusers is not compelling.
MECHANISM OF ACTION
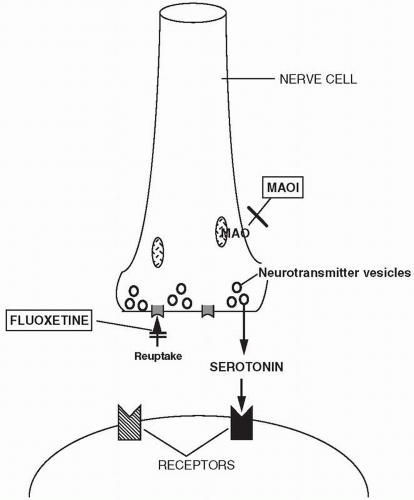
The precise mechanisms by which the antidepressant drugs exert their therapeutic effects remain unknown, although much is known about their initial actions within the nervous system. All of the currently marketed antidepressants interact with the monoamine neurotransmitter systems in the brain, particularly the norepinephrine and serotonin systems and to a lesser extent the dopamine system. Essentially all currently marketed antidepressants have as their molecular targets components of monoamine synapses, including the reuptake transporters (that terminate the action of norepinephrine, serotonin, or dopamine in synapses), monoamine receptors, or enzymes that serve to metabolize monoamines. What
remains unknown is how these initial interactions produce a therapeutic response often after a latency of weeks. The search for the molecular events that convert altered monoamine neurotransmitter function into the lifting of depressive symptoms is currently a matter of intense research.
The architecture of monoamine neurotransmitter systems in the brain is based on the synthesis of the neurotransmitter within a restricted number of nuclei within the brainstem with neurons projecting widely throughout the brain and, for norepinephrine and serotonin, the spinal cord as well. Norepinephrine is synthesized within a series of nuclei in the medulla and pons, of which the largest is the nucleus locus ceruleus. Serotonin is synthesized in the brainstem raphe nuclei. Dopamine is synthesized in the substantia nigra and the ventral tegmental area of the midbrain. Through extensive projection networks, these neurotransmitters influence a large number of target neurons in the cerebral cortex, basal forebrain, striatum, limbic system, and brainstem where they interact with multiple receptor types to regulate arousal, vigilance, attention, sensory processing, emotion, and cognition (including memory).
One of the classic animal models of depression uses the drug reserpine, which depletes neurons of monoamine neurotransmitters, including norepinephrine, serotonin, and dopamine, and causes hypomotility. Similarly, reserpine was believed to induce depression in some humans (although this is a matter of some dispute), which may be clinically indistinguishable from major depressive illness. In animal models, the cyclic antidepressants are partly able to reverse the hypomotility induced by reserpine and other amine-depleting agents, such as tetrabenazine.
Norepinephrine, serotonin, and dopamine are removed from synapses after release by reuptake, mostly into presynaptic neurons. This mechanism of terminating neurotransmitter action is mediated by specific norepinephrine, serotonin, and dopamine reuptake transporter proteins. After reuptake, norepinephrine, serotonin, and dopamine are either reloaded into vesicles for subsequent release or broken down by the enzyme monoamine oxidase (MAO). MAO is present in two forms (MAOA and MAOB), which differ in their substrate preferences, inhibitor specificities, tissue expression, and cell distribution. MAOA preferentially oxidizes serotonin and is irreversibly inactivated by low concentrations of the acetylenic inhibitor clorgyline. MAOB preferentially oxidizes phenylethylamine and benzylamine and is irreversibly inactivated by low concentrations of pargyline and deprenyl. Dopamine, tyramine, and tryptamine are substrates for both forms of MAO. Catecholamines are also broken down by catechol-O-methyltransferase, an enzyme that acts extracellularly. Two common alleles of catechol-O-methyltransferase have been identified, resulting in high and low enzyme activity. There is now research attempting to determine whether these alleles contribute to risk of mental disorders or to interindividual responses to drugs.
The TCAs and other cyclic antidepressants, as well as the SNRIs, block the reuptake of norepinephrine and serotonin in varying ratios, thus potentiating their action (
Fig. 3.1). The TCAs doxepin, amitriptyline, and nortriptyline also inhibit glycine uptake by equally blocking the glycine transporters 1b (GLYT1b) and 2a (GLYT2a). The cyclic antidepressant amoxapine is a selective inhibitor of GLYT2a. Whether these properties are relevant to antidepressant action is unclear. Amoxapine is also a dopamine D
2 receptor antagonist
in vivo, although, interestingly,
in vitro data suggest that trimipramine and clomipramine have comparable affinity for the dopamine D
2 receptor. There is concern that amoxapine, therefore, could exhibit neuroleptic-like side effects. TCAs, to varying degrees, are also fairly potent blockers of histamine H
1 receptors, serotonin 5-HT2 receptors, muscarinic acetylcholine receptors, and α
1-adrenergic receptors. All the available SNRIs (venlafaxine, duloxetine, and milnacipran) share the property of being relatively potent inhibitors of serotonin and norepinephrine uptake with minimal
affinity for postsynaptic receptors, with the exception of venlafaxine, which acts as a mild antagonist of nicotinic cholinergic receptors.
At therapeutically relevant doses, the SSRIs exhibit significant effects primarily on serotonin reuptake in the human brain. The SSRIs also appear to have effects at other monoamine transporters that vary from agent to agent, with sertraline demonstrating modest dopamine reuptake inhibition and paroxetine demonstrating modest norepinephrine reuptake inhibition. In addition, fluoxetine, particularly the R-isomer, has mild 5-HT2A and 5-HT2C antagonist activity, as well as weak noradrenergic reuptake inhibition. SSRIs have minimal or no affinity for muscarinic cholinergic receptors with the exception of paroxetine, which is a weak cholinergic receptor antagonist; SSRIs also have negligible effects on histaminergic and adrenergic receptors. The lack of significant action on these receptors contributes the mild side-effect profile of SSRIs compared with TCAs.
At therapeutically relevant doses, the NRIs have significant effects primarily on norepinephrine reuptake, although the NRI atomoxetine is also a weak inhibitor of serotonin uptake. The NRI reboxetine appears to have antagonist properties at nicotinic cholinergic receptors.
MAOIs potentiate the action of biogenic amines by inhibiting MAO activity, thus blocking the intracellular catabolism of monoamines. The MAOIs available in the United States are all irreversible inhibitors of MAOA and MAOB activity. Newer MAOIs, such as brofaromine and moclobemide, reversibly inhibit MAOA activity. More recently, additional pharmacologic properties for the MAOIs have been revealed. MAOIs, for instance, also appear to inhibit the binding of [3H] quinpirole, a dopamine agonist with high affinity for D2 and D3 dopamine receptors. To complicate the pharmacology of MAOIs, two of the MAOIs, selegiline and tranylcypromine, have methamphetamine and amphetamine as metabolites; phenelzine and its yet unidentified metabolite also elevate brain gammaaminobutyric acid levels. R(−)-selegiline but not S(+)-selegiline also appears to induce dopamine release by directly modulating ATP-sensitive potassium channels, and the (−) enantiomer of tranylcypromine also appears to inhibit catecholamine uptake.
Serotonin receptor agonists and antagonists primarily bind to serotonin 5-HT2 receptors. The serotonin receptor antagonists (nefazodone and trazodone) primarily block serotonin 5-HT2A receptors (in some cases, demonstrating partial agonist properties) and share a rather complex pharmacology. Findings from in vitro and in vivo studies support the hypothesis that m-chlorophenylpiperazine (m-CPP), an active metabolite of both trazodone and nefazodone, releases neuronal 5-HT via a nonexocytotic carrier-mediated exchange mechanism involving 5-HT transporters. In addition, both trazodone and nefazodone are relatively weak inhibitors of serotonin and norepinephrine uptake, and trazodone appears to stimulate µ-opioid receptors and is a potent agonist of the serotonin 5-HT2C receptors, which are able, when activated, to inhibit the N-methyl-D-aspartate-induced cyclic guanosine monophosphate elevation. Both trazodone and, to a lesser degree, nefazodone block α1-adrenergic receptors. Buspirone and gepirone act as full agonists at the serotonin 5-HT1A autoreceptors and are generally, although not exclusively, partial agonists at postsynaptic serotonin 5-HT1A receptors. Both compounds also have weak affinity for the α1-adrenoceptors and have mild dopamine D2 antagonism. Buspirone also has α2-adrenoceptor antagonist properties via its principal metabolite, 1-(2-pyrimidinyl)-piperazine.
The NDRIs primarily block the reuptake of dopamine and norepinephrine and have minimal or no affinity for postsynaptic receptors. Although bupropion has been recently characterized by some researchers as an NDRI, both bupropion and its metabolite S,S-hydroxybupropion display only weak affinity for the dopamine transporter, and only S,S-hydroxybupropion appears to possess measurable affinity at the norepinephrine transporter level. In addition, other researchers have argued that the NDRI bupropion’s effect on norepinephrine is primarily through an increase in presynaptic release. Therefore, one may argue that bupropion’s effects on both norepinephrine and dopamine neurotransmission may be due to mechanisms other than those of pure NDRIs. It also appears that bupropion is able to antagonize α3β2 and α3β4 nicotinic cholinergic receptors.
The α2-adrenergic receptor antagonists (e.g., mirtazapine, mianserin) appear to enhance the release of both serotonin and norepinephrine by blocking autoand hetero-α2-receptors. Because mirtazapine appears to be a blocker of serotonin 5-HT2 and 5-HT3 receptors as well, it is thought to enhance the release of norepinephrine and enhance 5-HT1A-mediated serotonergic transmission. Mirtazapine is also a potent histaminergic H1-receptor antagonist and is more sedating than the SSRIs. Mianserin is also a 5-HT2 antagonist.
Because TCAs and MAOIs were the first antidepressants to be introduced in the market, this was initially interpreted as suggesting that antidepressants work by significantly increasing noradrenergic or serotonergic neurotransmission, thus compensating for a postulated state of relative deficiency. However, this simple theory could not and cannot fully explain the action of antidepressant drugs for a number of reasons. The most important of these includes the lack of convincing evidence that depression is characterized by a state of inadequate monoamine neurotransmission. In fact, the results of studies testing the monoamine depletion hypothesis in depression have yielded inconsistent results. Moreover, blockade of reuptake by the cyclic antidepressants and SSRIs and inhibition of MAO by MAOIs occur rapidly (within hours) after drug administration, but antidepressants are rarely clinically effective prior to 2 weeks and may require 6 weeks or more.
These considerations have led to the idea that inhibition of monoamine reuptake or inhibition of MAO by antidepressants represents an initiating event. The actual therapeutic actions of antidepressants, however, result from slower adaptive responses within neurons to these initial biochemical perturbations. Research investigating slow-onset changes in neurons that might better reflect the time course of antidepressant action is ongoing. It has been found, for example, that chronic (2 weeks) treatment of rats with cyclic antidepressants or MAOIs is associated with a reduction in number (downregulation) of β1-adrenergic receptors, accompanied by a decreased activation of adenyl cyclase by norepinephrine. Many antidepressants also downregulate α2-adrenergic receptors and have variable effects on 5-HT2 serotonin receptors. Changes in receptor number are currently seen as correlates of long-term administration (established largely in normal rat brain), not a likely therapeutic mechanism. Slow-onset changes in the nervous system that may be related convincingly to the mechanism of action of antidepressants are actively being sought. It is now well established that antidepressants, like other drugs that affect neurotransmitters, alter the expression of a large number of genes in the brain. The question is which genes are relevant and what is the effect of increasing or decreasing the levels of the proteins that they encode. For example, increased neurogenesis in areas of the brain, such as the hippocampus, is considered one of the possible downstream effects of antidepressants.
Although research to understand the therapeutic actions of antidepressants has been challenging, receptor studies have been useful in understanding some of their side effects. For example, the rank order of binding affinities of cyclic antidepressants at muscarinic cholinergic receptors generally parallels the potency of their clinical anticholinergic effects. Similarly, high affinities for histamine H1 receptors may partially explain their strong sedative effects and their ability to increase appetite, while high affinities for α1-adrenergic receptors may be related to the risk of orthostatic hypotension. Such information is very useful for clinicians to understand and treat side effects and to those attempting to develop new antidepressants.
Given that antidepressants that act via monoamine systems have similar efficacy for MDD, attempts have been made to find antidepressant compounds that act independently of monoamines. For example, attempts are being made to develop corticotropin-releasing factor 1 receptor antagonists and drugs that block the neurokinin 1 receptors (NK-1 antagonists) to treat depression.
CLINICAL USES
As one would expect, the newer antidepressants (SSRIs, SNRIs, NRIs, NDRIs, and serotonin receptor antagonists) all have safety and side-effect advantages over the TCAs and MAOIs. Since the introduction of fluoxetine, the SSRIs and SNRIs have become the most often prescribed initial treatment for MDD. The success of the SSRIs and SNRIs in displacing tricyclic drugs as first-choice agents is not based on
established differences in efficacy but rather on a generally more favorable side-effect profile such as lack of anticholinergic and cardiac side effects and a high therapeutic index (ratio of lethal dose to therapeutic dose), combined with ease of administration. Furthermore, with certain comorbidities of depression, such as OCD, SSRIs offer advantages in efficacy over the tricyclics. Some of the metaanalytic studies of the efficacy of SSRIs and TCAs have even concluded that some of the tertiary amine tricyclics may be slightly more efficacious than SSRIs in more severe, melancholic depression. Because of their toxicity and risk, MAOIs are a class of drugs reserved for patients for whom other treatments have failed.
Suicide Risk
The tricyclic and related cyclic antidepressants (maprotiline and amoxapine) and the MAOIs are potentially lethal in overdose, unlike the SSRIs, the SNRIs, and the other newer antidepressants. Thus, a careful evaluation of impulsiveness and suicide risk influences not only the decision as to the need for hospitalizing a person with depression but also the choice of an antidepressant. For potentially suicidal or highly impulsive patients, the SSRIs, the SNRIs, and the other newer agents would be a better initial choice than a cyclic compound or an MAOI. Patients at elevated suicide risk who cannot tolerate these safer compounds or who do not respond to them should not receive large quantities or refillable prescriptions for TCAs or MAOIs. Generally, patients who are new to treatment or those at more than minimal risk for suicide whose therapeutic relationship is unstable should receive a limited supply of any medication.
Evaluation for suicide risk must continue even after the initiation of treatment. Although suicidal thoughts are often among the first symptoms to improve with antidepressant treatment, they may also be slow to respond to treatment, and patients may become demoralized before therapeutic efficacy is evident. Side effects such as agitation and restlessness and, most important, intercurrent life events may exacerbate suicidal thoughts prior to a full therapeutic response. Thus, rarely, for a variety of reasons, patients may temporarily become more suicidal following the initiation of treatment. Should such worsening occur, appropriate interventions may include management of side effects, more frequent monitoring, discontinuation of the initial treatment, or hospitalization. The FDA has asked manufacturers of almost all the antidepressant drugs to include in their labeling a warning statement that recommends close observation of adult and pediatric patients treated with these drugs for worsening depression or the emergence of suicidality. This warning was based on the analyses of clinical trials data comparing the relative risk of emergence of suicidal ideation in patients on these drugs and placebo following initiation of treatment. The difference was small (less than twofold) but statistically significant. This finding underscores the need for good practice, which includes education of patients (and families if the patient is a child) about side effects of drugs (including the possible emergence of suicidal thoughts and behaviors), close monitoring (especially early in treatment), and the availability of a clinician in case suicidality emerges or worsens. Further analyses from the FDA on the relative risk of treatment-emergent suicidal ideation with the SSRIs have demonstrated that a very small increase in risk is observed only in adolescents and young adults. A consensus remains, however, that the risks associated with withholding antidepressant treatment from patients, including pediatric patients, with serious depression outweighs the risks associated with the drugs.
Treatment-Resistant Depression
A large number of patients respond
partially or not at all to an initial antidepressant trial. When evaluating possible causes for antidepressant nonresponse, it is important to ensure that the initial diagnosis is correct and that there is no
unsuspected comorbid psychiatric or medical condition (e.g., alcoholism or thyroid disease) negatively affecting treatment response. There are three general strategies for treating resistant depression that can be used in an orderly fashion (these strategies are discussed in detail for each specific class of drug):
1. Optimization/dose increase—ensuring adequate drug doses for the individual, which may be higher than initial doses (e.g., fluoxetine, 40 to 80 mg; desipramine, 200 to 300 mg) and adequate duration of treatment (8 to 12 weeks or longer). In addition, the possibility of failure of the patient to adhere to the regimen, which occurs more commonly than most practitioners appreciate, should be considered. Dose increases above the FDA-recommended dose range may occur in clinical practice when patients have partial or nonresponse in the absence of side effects. In some cases, obtaining blood levels of the antidepressant (typically 24 hours after the last dose) may help and guide this decision.
2. Augmentation or combination—addition of drugs (to the ongoing treatment) that are not antidepressant agents themselves is termed augmentation therapy; well-studied augmentation strategies included, for example, adjunctive second-generation antipsychotic agents, lithium, or Ltriiodothyronine (T3). Other less well-studied augmentation strategies include the use of buspirone, modafinil, psychostimulants, methylfolate, and S-adenosyl-methionine (SAMe). Combination treatment generally refers to the prescribing of more than one antidepressant. Examples of this approach include adding antidepressants such as bupropion or mirtazapine to SSRIs or SNRIs. The array of putative augmentation and combination strategies has dramatically increased with the newer antidepressant agents. Although there are many commonly used augmentation and combination strategies, few have been well studied and supported by clinical research.
3. Switching—change in the primary drug. If lack of efficacy was the problem, a switch to an agent from a different drug class is reasonable, particularly when the first drug is poorly tolerated; for example, if the first drug was an SSRI, switch to bupropion or to an SNRI. If, however, the first drug failed because of side effects, a drug within the initial class may be effective if it is tolerated. For reasons that are unclear but likely reflect the minor pharmacological differences between SSRIs, a switch within the class is clinically helpful for primary efficacy failures sufficiently often to warrant a second SSRI trial for some patients before switching out of class. If the patient remains seriously depressed despite additions or changes in medication, consideration should be given to the relative risks of additional trials (based on severity of symptoms and concern about time delay) versus the use of ECT.
Continuation and Maintenance Treatment
Originally based on studies with TCAs, patients with unipolar depressive disorders were observed to be at high risk for relapse when treatment was discontinued within the first 16 weeks of therapy. Therefore, in treatment responders, most experts favor a continuation of antidepressant therapy for a minimum period of 6 months following the achievement of remission. The value of continuation therapy for several months to prevent relapse into the original episode has also been established for virtually all the newer agents. Risk of recurrence after this 6- to 8-month continuation period, that is, the development of a new episode after recovery from the index episode, is particularly elevated in patients with a chronic course before recovery, residual symptoms, and multiple prior episodes (three or
more). For these individuals, the
optimal duration of maintenance treatment is unknown but is often measured in years. Based on research to date, prophylactic efficacy of an antidepressant has been observed for as long as 5 years with clear benefit. In contrast to the initial expectation that maintenance therapy would be effective at dosages lower than that required for acute treatment, the current consensus is that full-dose therapy is required for effective prophylaxis. In some cases, adequate maintenance may actually require doses of antidepressants higher than those that were acutely effective and therefore some dose flexibility.
Two of the main issues in the use of antidepressants for continuation and maintenance treatment are the persistence of troublesome side effects emerging during the acute phase of treatment and the emergence of side effects during the continuation and maintenance phases. For example, continuation and maintenance therapy with TCAs is often difficult because of early-onset, persisting side effects such as weight gain, dry mouth, and constipation. With the newer drugs, in particular the SSRIs, the main issue is often the emergence of certain side effects during the continuation and maintenance therapy such as apathy, cognitive side effects, sexual dysfunction, weight gain, fatigue, and sleepiness.
About 20% to 30% of patients who are treated with each of the classes of antidepressants will experience a return of depressive symptoms despite continued treatment. In such patients, a dose increase of the antidepressant is typically the first-line approach, although the same considerations and strategies described for treating resistant depression are also of potential value.
Except for amoxapine, which possesses some antipsychotic drug properties and has been implicated in tardive dyskinesia, there are no known severe adverse effects specifically due to long-term antidepressant treatment, except that the risk of a discontinuation syndrome with TCAs, MAOIs, SSRIs, and SNRIs is more likely after abrupt interruption of chronic treatment especially with shorter half-life agents. Discontinuation syndromes are typically characterized by the emergence of transient, short-lived physical (e.g., dizziness, headache, nausea, flulike symptoms) and psychological (e.g., dysphoria, irritability, anxiety) symptoms that abate rapidly on reintroducing the antidepressant agent. To minimize the risk for discontinuation reactions, it is always advisable to gradually taper and discontinue antidepressants after chronic exposure.
CHOICE OF ANTIDEPRESSANT
A large number of antidepressants are available (
Table 3.3), including SSRIs, SNRIs, tricyclic and related compounds, MAOIs, NRIs, NDRIs, serotonin receptor antagonists and agonists (e.g., nefazodone and trazodone), and the α
2-adrenergic receptor antagonists. Successful use of antidepressants requires the following:
1. Good patient selection as determined by a thorough and comprehensive diagnostic evaluation. In particular, attention should be paid to comorbid psychiatric and medical disorders.
2. Choice of a drug with an acceptable side-effect profile for the given patient.
3. Adequate dosage. In the absence of side effects and response, dose escalations within the recommended range should be pursued aggressively.
4. Drug trial of at least 6 to 12 weeks for MDD and other unipolar depressive disorders.
Many patients with potentially treatable depression fail to improve because of inadequate dosing or duration of treatment or both. Every physician does not need to be thoroughly familiar with every antidepressant on the market, but it is useful for the clinician to be comfortable prescribing several drugs that differ in mechanism and side-effect profile. The most important considerations in choosing among these drugs are efficacy for the condition being treated and side effects. The
efficacy of the available antidepressants for MDD, including various subtypes, and for other disorders has been described previously. Although there are some differences in efficacy across the class of antidepressants for subtypes of depression, the major clinically significant differences among the antidepressants are in their side effects. All of the TCAs and related compounds (maprotiline and amoxapine) cause some degree of anticholinergic side effects and postural hypotension, and all are potentially cardiotoxic in susceptible individuals or in overdose (
Table 3.4). The consensus among experts in formulating depression treatment guidelines is that the first line of treatment should be a newer antidepressant in light of safety and tolerability concerns over the long term. Although most of the newer antidepressants may initially cause agitation, insomnia, nausea, and headache, and, over time, sexual dysfunction, among other side effects, they are generally more tolerable to patients than are the older cyclic compounds. The MAOIs may cause significant side effects, including postural hypotension, and require dietary and drug interaction precautions, thereby raising issues of treatment adherence.
At a minimum, general physicians should be comfortable prescribing at least two of the SSRIs and the SNRIs, and at least one compound of every other class of new antidepressants (NRIs, NDRIs, serotonin receptor antagonists and agonists, and α2-adrenergic receptor antagonists). Because psychiatrists will often be called on to treat patients who have failed initial treatments, they should have broader experience, including experience with tricyclics and MAOIs. The following are general guidelines for choosing an antidepressant:
1. It is reasonable to prescribe a drug that was clearly effective in the past if it was well tolerated by the patient. It is also reasonable to prescribe an antidepressant that was clearly effective in the past in a first-degree relative of the patient.
2. Avoid drugs (e.g., amitriptyline, protriptyline) with the highest levels of anticholinergic activity to maximize patient comfort and compliance. In addition to bothersome side effects such as dry mouth, dry throat, urinary retention, blurred vision, and constipation, diminished working memory and dental cavities are also considered to be possible side effects secondary to anticholinergic effects. (Despite its relatively high anticholinergic potency, clomipramine is considered useful because of its efficacy in OCD and its superior efficacy for severe and melancholic depression.)
3. For patients with initial insomnia, many clinicians may opt for the temporary use of a short-acting benzodiazepine or other hypnotic combined with SSRIs, SNRIs, or other nonsedating newer antidepressants, with the expectation of tapering and discontinuing the hypnotic when the depression has improved. Both benzodiazepines and nonbenzodiazepine hypnotics such as zolpidem and eszopiclone have been shown to be more effective than placebo in treating insomnia during antidepressant treatment. Benzodiazepines with relatively long half-lives increase the risk of unwanted somnolence and sleepiness during the day. Other clinicians may select a sedating secondary-amine tricyclic compound (e.g., nortriptyline) given at bedtime. To avoid anticholinergic and cardiovascular side effects, however, the sleep-enhancing, α2-adrenergic receptor antagonist mirtazapine would be preferred, with the expectation that daytime sedation will abate over time with these medications. The sedating tricyclic drug amitriptyline used to be popular with general physicians, but because it is among the most anticholinergic of the tricyclics, it should not be a first-choice agent. The serotonin receptor antagonist trazodone, which lacks anticholinergic side effects, is very sedating, but its overall efficacy as monotherapy for depression is often questioned by clinicians. Trazodone at lower doses (50 to 300 mg at bedtime) has been used in place of benzodiazepines or other hypnotics to treat insomnia, particularly middle to late insomnia, in patients treated for depression with an SSRI or an SNRI. Although benzodiazepines and hypnotics such as zolpidem are often started either simultaneously with the antidepressant or added later to the treatment, trazodone is typically not started at the same time as the antidepressant but is instead added later on for the management of the insomnia. For most depressed patients, if insomnia is related to depression, the sleep difficulties will improve with any effective antidepressant over time, even those without sedation as a side effect. With more sedating drugs, on the other hand, the side effect may persist after it is helpful and may interfere with daytime function and compliance.
4. All of the tricyclics and most of the SSRIs (e.g., fluoxetine, fluvoxamine, paroxetine, citalopram, and sertraline) are available generically and have the advantage of being the least costly treatments in terms of formulary cost. On the other hand, when one considers the other direct and indirect costs of treatment, however, the financial savings of the generic TCAs diminish.
5. For patients who want to avoid sedation, it is reasonable to prescribe SSRIs, SNRIs, NRIs, and NDRIs, which are usually nonsedating. Among the tricyclics, desipramine and protriptyline are probably the least sedating.
6. In elderly patients, especially those with constipation or glaucoma, and in men with prostate hypertrophy, the least anticholinergic drugs should be used, such as SSRIs or other newer agents.
7. SSRIs, SNRIs, NRIs, NDRIs, and the α
2-adrenergic receptor antagonist mirtazapine generally do not cause postural hypotension, whereas TCAs, MAOIs, and the serotonin receptor antagonists nefazodone and trazodone
have this risk. In the case of TCAs and both nefazodone and trazodone, the blockade of α
1-receptors is thought to be related to the risk of postural hypotension. Nortriptyline, desipramine, and protriptyline may have an advantage among the tricyclics in causing relatively less postural hypotension than the others. The tricyclics have on rare occasion been associated with hypertension, and at higher doses, a small percentage of patients may have blood pressure elevation with venlafaxine.
8. In patients with cardiac disease or significant delay in intracardiac conduction, the tricyclics with their quinidine-like properties should be avoided.
9. Epileptic patients may develop a primary depressive disorder or secondary depression. Because all of the tricyclic and related cyclic antidepressants and the NDRI bupropion may decrease the seizure threshold, these agents should be avoided in these populations. When combining any antidepressant with an anticonvulsant, the clinician must be alert for possible pharmacokinetic interactions, because many of the anticonvulsants may be inducers or inhibitors of cytochrome P450 (CYP) systems.
10. Most antidepressants may cause or worsen sexual dysfunction. In particular, decreased libido, delayed orgasm or anorgasmia, arousal difficulties, and erectile dysfunction have been reported with almost all the classes of antidepressants. Trazodone and nefazodone have been associated with the uncommon occurrence of priapism in both men and women. Two recent studies by Clayton et al. suggest that the NDRI bupropion and the NRI reboxetine may be the least likely antidepressants to cause sexual dysfunction.