SCHIZOPHRENIA
Schizophrenia is a chronic psychotic illness that typically begins early in adulthood. Some cases begin with a prodromal period of several years, characterized by nonspecific symptoms (depression, social withdrawal, and subtle perceptual changes), that is followed by the eventual development of acute, frank psychosis (i.e., hallucinations, delusions, or disorganized thinking or behavior). Other cases have a more abrupt onset of psychosis. Often, the illness course shows periods of less florid psychosis, punctuated by acute exacerbations. Unfortunately, the overall course of this illness generally leads to a diminished plateau of function and an inability to maintain an active and productive life. Symptoms of schizophrenia are often divided into positive symptoms (i.e., symptoms that are not present in normal human cognitions, perceptions, or affect, such as hallucinations, delusions, and thought disorder), negative or deficit symptoms (i.e., loss of qualities normally present in healthy individuals, such as impoverishment of thought, blunted affect, and lack of initiative and motivation), and cognitive symptoms (particularly impairment in executive function and verbal memory). The cognitive impairment and the negative symptoms are essential features of the illness in that they underlie the poor functioning that is typical for many patients.
Many patients also have co-occurring anxiety disorders (e.g., obsessive-compulsive disorder or panic disorder) and depression. Suicide is the leading cause of death. Substance abuse occurs in approximately half of the patients. These co-occurring disorders worsen the course of the illness and complicate treatment. Stimulant use and alcohol binges often lead to psychosis exacerbation. Nicotine dependence is highly prevalent, and while cigarette smoking may help improve attention (by partially correcting the so-called sensory gating deficit) and provide some pleasure, it clearly contributes to cardiovascular and pulmonary diseases frequently experienced by these patients.
Patients with schizophrenia often neglect their health and avoid getting medical and dental treatment. They frequently have untreated medical conditions, including hypertension, coronary artery disease, diabetes, chronic obstructive pulmonary disease, and dyslipidemia. Unfortunately, even when patients have laboratory evidence of medical conditions such as diabetes, they frequently go undertreated or untreated. Patients with schizophrenia die young—10 to 15 years younger than the general population, on average. Cardiovascular disease is the most common medical cause of death. Several of the second-generation antipsychotics, unfortunately, may contribute to cardiovascular morbidity because of their propensity to cause weight gain, dyslipidemia, and insulin resistance.
Even though antipsychotics are critical for illness treatment, these drugs do not treat the full syndrome of schizophrenia, and rarely does drug treatment alone lead to patients regaining their premorbid function. Antipsychotics are frequently helpful for the positive symptoms of schizophrenia, but they provide only limited to no benefit for primary negative and cognitive symptoms. Other psychosocial treatments including social skills and vocational training, case management, and cognitive behavioral therapy are necessary adjuncts to achieve the best possible satisfactory outcomes.
OTHER PRIMARY PSYCHOTIC DISORDERS
In addition to schizophrenia, several other closely related psychotic conditions such as schizophreniform disorder, schizoaffective disorder, or delusional disorder may be amenable to antipsychotic treatment. Schizoaffective disorder probably represents a heterogeneous group of diseases rather than a single disease entity. The diagnosis is applied to patients who have periods of major manic or depressive symptoms or both but who have prominent psychotic symptoms which meet criteria for schizophrenia, even at times when they are relatively free of affective symptoms. Clinically, affective symptoms sometimes respond to lithium, antidepressants, or an anticonvulsant. Psychotic symptoms, especially those that occur between episodes of mood disorder, generally require treatment with antipsychotic medication. The dosages of antipsychotic medication for both acute florid symptoms and chronic maintenance are the same as those for the analogous stages of schizophrenia. All second-generation compounds are likely to be effective for schizoaffective disorder, although more study is warranted.
Schizophreniform disorder involves overt psychotic symptoms of less than 6 months’ duration, with return to the premorbid level of functioning. The onset of symptoms tends to be rapid, rather than insidious, and patients may demonstrate confusion or perplexity at the height of their syndrome. Many, but not all, patients lack the flat affect of typical schizophrenia. Schizophreniform patients represent a heterogeneous group. Depending on the population studied, some investigators predominantly find mood disorders represented, whereas others find heterogeneity, with perhaps half of them going on to manifest schizophrenia over time. The early stages of treatment of schizophreniform disorder are the same as for any acute psychosis (see later section on therapeutic use). Given that the long-term prognosis for recovery of many of these patients is good, after the first episode an attempt can be made to taper and discontinue antipsychotic drugs entirely if symptoms fully remit and remain in remission for 6 months to a year. Many patients who meet criteria for schizophreniform disorder will also benefit from lithium or anticonvulsants (see
Chapter 4) if symptoms of bipolar disorder emerge.
Delusional disorder entails the presence of delusions without hallucinations or language impairment. This condition is considerably less common than schizophrenia, and usually starts in middle age. The most common types include paranoid delusions, but some patients may experience delusional jealousy, erotomania, or somatic delusions. Patients with delusional disorder are often difficult to treat because of illness denial, and their pervasive suspiciousness often extends to physicians and medical treatment. As a result, they are often brought to treatment by others and are often nonadherent to treatment. If medication is introduced to the patient as a means of helping cope with anxiety, stress, or other complaints rather than explicitly confronting delusions, the initiation of treatment may be more acceptable. Nonetheless, over time, it is best if the patient can be helped to develop insight into the distortions and misperceptions.
Antipsychotic drugs are effective in some patients with delusional disorder, especially in those whose symptoms are of recent onset. As with other psychotic disorders, the agents of choice are the atypical antipsychotics. For patients with chronic, systematized delusions, the response rate may be lower than for those with a more recent onset. Pending systematic studies of this clinical population, dosing guidelines should follow those for treating schizophrenia, while considering the possibility that lower doses may be adequate for some patients. If first-generation drugs are tried, low doses should be used initially (e.g., haloperidol 2 to 5 mg per day or the equivalent) to minimize side effects and to enhance compliance. Consideration should be given to a trial of antidepressants or lithium if affective symptoms are apparent or if there is a family history of mood disorder.
GENERAL COMMENTS ABOUT ANTIPSYCHOTICS
The antipsychotic drugs are the cornerstone of treatment of schizophrenia and other psychotic disorders, such as schizoaffective disorder. Antipsychotic drugs have been in clinical use since the 1950s, when chlorpromazine, a phenothiazine derivative that was developed as an antihistamine, was found to have antipsychotic properties. The key observation was that chlorpromazine and later antipsychotic drugs were not acting as nonspecific sedatives but were ameliorating core psychotic symptoms such as hallucinations and delusions.
Chlorpromazine provided a model for the development of a wide variety of chemically distinct compounds effective for the psychoses, but all of these first-generation compounds (with the exception of clozapine) had a liability for causing extrapyramidal symptoms (EPS) by virtue of their major shared property, potent antagonism of the D2 dopamine receptor. In addition to their antipsychotic properties, these drugs have had other uses on the basis of their ability to block D2 dopamine receptors (e.g., as antiemetics and in palliation of some movement disorders characterized by excessive movement). The first-generation D2 antagonist antipsychotic drugs have come to be described as typical to contrast them with clozapine and with newer second-generation or so-called atypical drugs that have a reduced liability for EPS. The EPS burden also led to use of the term neuroleptic for these older drugs because these drugs could produce neurologic disorders that looked similar to Parkinson’s disease or dystonias. In addition, long-term use of these drugs, as is typically required in schizophrenia, posed a high risk of a permanent movement disorder, tardive dyskinesia (TD). Even in the short term, in addition to producing parkinsonian symptoms, first generation antipsychotic drugs produce side effects (e.g., akathisia or akinesia) that could mimic or exacerbate the symptoms for which the drugs were originally prescribed. In short, these older antipsychotic drugs were effective and indeed critically important in the treatment of psychotic disorders for more than 40 years, but at the price of serious motor system problems that could limit therapy. Although the liability for extrapyramidal neurologic side effects is an intrinsic liability of first-generation drugs, as a result of their selective blockade of D2 dopamine receptors, they were often also administered at excessive doses, which markedly exacerbated the problem.
The introduction of risperidone (Consta and Risperdal) in 1993 began a new era in the treatment of psychotic illnesses with the introduction and widespread adoption of a group of compounds that have a reduced liability for producing EPS. These have variously been referred to as “atypical” or
second-generation antipsychotic drugs. However, this change began with the reintroduction of clozapine, an older drug with very low risk for EPS liability and the greatest efficacy in schizophrenia. Clozapine had not been marketed because it carried approximately a 1% risk of potentially lethal agranulocytosis. Studies that demonstrated the drug’s not only very low EPS liability but also greater efficacy for treating schizophrenia than any other antipsychotic drug led to its reintroduction. Because of the risk of agranulocytosis, however, cumbersome and expensive weekly monitoring of white blood cell (WBC) counts became part of any clozapine regimen. In addition, clozapine has its own troublesome side effects (including sedation, weight gain, and a reduction in seizure threshold). However, the benefits of clozapine have been demonstrated to outweigh its risks for many individuals with schizophrenia who respond poorly to other treatments. Although it is still unclear what gives clozapine its enhanced efficacy (it has relatively low affinity for D
2 receptors compared with the other older drugs, exhibits potent antagonism of serotonin 5-HT
2A receptors, and interacts with many other receptors), it became a new, if partly mysterious, model for drug development. Since the reintroduction of clozapine, several newer compounds—risperidone, olanzapine (Zyprexa), quetiapine (Seroquel), ziprasidone (Geodon), and aripiprazole (Abilify)—have been shown to be effective for schizophrenia
and other psychoses and useful for mania as well, though, unlike clozapine, they are not superior to the older drugs. Because these drugs have reduced EPS liability, have a generally milder side-effect profile than does clozapine, and do not pose a risk of agranulocytosis, they have rapidly become the first-line drugs for the treatment of psychotic disorders. As a group, these drugs have been shown in well-controlled trials of greater than 4 to 20 weeks to be at least as effective as the older typical antipsychotic drugs, although they do not exhibit the clear efficacy advantages of clozapine in the most resistant cases. Moreover, many of these drugs have their own problematic side effects. Recent prospective studies showing similar effectiveness between the old and new drugs have made us question the enthusiasm once accompanying the second-generation antipsychotics.
As noted, a high affinity for D
2 dopamine receptors among the older compounds is clearly associated with their liability for producing EPS. However, the decreased liability for EPS in the newer compounds appears to reflect diverse mechanisms and is still not fully understood. For example, although risperidone has
high affinity for the D2 receptor, like haloperidol (
Table 2.1), its high affinity for the serotonin 5-HT
2A receptor may mitigate its EPS liability when it is given at lower doses (<6 mg per day). In contrast, quetiapine has a lower affinity for the 5-HT
2A receptor than haloperidol, but it also has a lower affinity for the D
2 dopamine receptor. As described, clozapine has a relatively low D
2 receptor affinity and a high affinity for the 5-HT
2A receptor, but it interacts with so many receptors (
Table 2.1) that the basis of its efficacy and atypical side-effect profile remains unclear.
Multiple clinical trials have shown that antipsychotic drugs are effective both for acute exacerbations of schizophrenia and for long-term maintenance. Rigorous studies of clozapine have shown that it may be of particular benefit in chronic schizophrenia refractory to other antipsychotic drugs; case series and anecdotal reports suggest that it also may be effective in refractory atypical psychoses, such as schizoaffective disorder. Clinical trials have not convincingly shown superior efficacy of second-generation antipsychotics, other than clozapine, in schizophrenia when the comparator, most often haloperidol, is administered at appropriately low doses and combined with anticholinergic agents. Use of high doses of haloperidol in several comparison trials creates conditions for newer drugs looking better than they may be.
It has often been stated that traditional antipsychotic drugs are more effective in treating positive symptoms than negative symptoms. However, when examined
carefully, this generalization is not entirely accurate in that those negative symptoms that occur during an acute exacerbation of schizophrenia often respond well to antipsychotic drugs. Moreover, many patients continue to have some residual positive symptoms, such as hallucinations and delusions, despite the use of antipsychotic medication. The expectation that all positive symptoms should respond to antipsychotic therapy has led to the use of excessive doses in some patients.
Negative symptoms that characterize the patient’s chronic course (i.e., negative symptoms that are present even at times when positive symptoms are minimal) tend to be relatively refractory to antipsychotic drug treatment and appear somewhat more responsive to clozapine. No existing drugs appear to ameliorate executive dysfunction and other core cognitive deficits like impaired verbal memory. These symptoms ultimately contribute substantially to disability even when other symptoms improve.
It is also important to recall that side effects of first generation antipsychotic drugs can mimic both positive and negative features of schizophrenia. Akathisia can be indistinguishable from agitation and anxiety (positive symptoms), and EPS effects of antipsychotics (e.g., bradykinesia, akinesia, and masked facies) can masquerade as negative symptoms of the disorder. Indeed, the D2 dopamine receptor antagonism of antipsychotic drugs, by causing even subtle akinesia, may create a therapeutic ceiling effect vis-à-vis negative symptoms in some patients.
CHEMISTRY
Phenothiazines, the first chemical class of antipsychotic drugs developed, are tricyclic molecules. Three subtypes of phenothiazines are available: aliphatics, piperidines, and piperazines. Those phenothiazines with aliphatic side chains (e.g., chlorpromazine) tend to be low-potency compounds (i.e., higher doses are needed to achieve therapeutic effectiveness). Piperidine substitutions impart anticholinergic properties and a lower incidence of EPS (e.g., thioridazine, mesoridazine). Piperazine phenothiazines (e.g., perphenazine, trifluoperazine, fluphenazine) are among the most potent antipsychotic molecules.
The thioxanthene class of antipsychotic drugs is chemically similar to the phenothiazines. The butyrophenones represent a class of extremely potent antipsychotic drugs. Of these, only haloperidol is currently approved for psychiatric use in the United States. Droperidol, a shorter-acting butyrophenone, is approved for use as a preanesthetic agent.
Several other compounds of varied chemical structures have been approved for the treatment of psychotic and other illnesses in the United States. Pimozide, a diphenylbutylpiperidine approved for Gilles de la Tourette syndrome (but not necessarily superior in efficacy to other antipsychotic drugs for this indication), is also a potent antipsychotic drug with a very long half-life (several days). In addition, there are compounds called dibenzodiazepines that closely resemble the tricyclic antidepressants with a seven-member central ring and a piperazine substitution; this class of antipsychotic drug is represented by the typical antipsychotic drug loxapine, as well as by the atypical drug clozapine. Risperidone is a benzisoxazole derivative that combines high affinity for D
2 dopamine receptors and 5-HT
2 serotonin receptors. Olanzapine is a thienobenzodiazepine agent with greater affinity for the serotonin 5-HT
2 receptors than for dopamine receptors and that, compared with other second-generation antipsychotics, is most like clozapine in its receptor affinities. Quetiapine is a dibenzothiazepine derivative with low affinity for serotonin receptors but weaker activity at dopamine receptors and multiple other receptors. Ziprasidone is a benzisothiazolyl piperazine derivative with greater affinity for serotonin 5-HT
2A receptors than for dopamine receptors but higher affinity for D
2 dopamine receptors than does clozapine or quetiapine. In this regard, it is similar to risperidone. Ziprasidone also has relatively low affinity for H
1 histamine receptors and α
1-norepinephrine receptors, which limits its liabilities for sedation and orthostatic hypotension. Aripiprazole is a quinolinone derivative, which like risperidone and ziprasidone, shows higher affinity for serotonin 5-HT
2A receptors than for dopamine receptors. In addition, aripiprazole appears to have partial agonist activity at D
2 dopamine receptors, although the clinical significance of this effect remains unknown.
PHARMACOLOGY
Potency Versus Efficacy
The distinction between potency and efficacy is helpful to an understanding of the pharmacology of antipsychotic drugs.
Efficacy refers to the therapeutic
benefits that can be achieved by a drug, whereas
potency describes the
amount of the drug needed to achieve the therapeutic effect. All of the first-generation antipsychotic drugs are equivalent in efficacy, meaning that at an optimal dosage, which differs for each drug (
Table 2.2), each of the older drugs has been found to be equally efficacious in treating psychotic disorders. A useful generalization about the older antipsychotic drugs is that those with low potency (which means that they must be given in higher doses) tend to be more sedating, tend to be more anticholinergic, and tend to cause more postural hypotension than the high-potency drugs. The high-potency drugs tend to cause more EPS.
Clozapine is the only drug that has convincingly shown greater efficacy than the older drugs. A pivotal trial conducted in patients with schizophrenia who had been unresponsive to at least two different antipsychotic drugs found significant improvement (defined as a modest 20% reduction in the rating scale) in 30% of 126 patients treated with clozapine for 6 weeks compared with only 5% of 141 patients treated with chlorpromazine. Clinical experience and meta-analyses have amply confirmed the results of this well-designed trial; that is, clozapine may effectively treat patients who do not respond to other antipsychotic drugs. For other second-generation drugs, there are both positive and negative trials showing greater efficacy than haloperidol in treatment-refractory patients, but the margin of superiority, if it exists, is not great. The only clear benefit of the second-generation drugs is the relative lack of EPS.
Absorption and Distribution
Traditional antipsychotic drugs are available for both oral and parenteral use, whereas second-generation drugs until recently have only been available for oral use. Parenteral short-acting formulations of second-generation antipsychotic drugs are available for ziprasidone, aripiprazole, and olanzapine and may become available for others (
Table 2.3). The pharmacokinetics of the first-generation drugs are well understood only for a few (especially chlorpromazine, thioridazine, and haloperidol), because of the complexity of active and inactive metabolites. Taken orally, the drugs are absorbed adequately, although somewhat variably. Food or antacids may decrease absorption.
Ziprasidone, however, is to be
taken with food, which doubles the amount of absorbed drug. Liquid preparations are absorbed more rapidly and reliably than tablets. There is a marked first-pass effect through the liver with oral administration (i.e., a high percentage of the drug is metabolized as it passes through the hepatic portal circulation). The peak effect of an oral dose generally occurs within 2 to 4 hours.
Parenterally administered antipsychotics are rapidly and reliably absorbed. Drug effect is usually apparent within 15 to 20 minutes after intramuscular (i.m.) injection, with peak effect occurring within 30 to 60 minutes. With intravenous (i.v.) administration, some drug effect is apparent within minutes, and peak effect occurs within 20 to 30 minutes. [The i.v. administration of antipsychotic drugs has
not been approved by the Food and Drug Administration (FDA). The haloperidol-like drug droperidol is approved for i.v. use for perioperative nausea and vomiting, although it is not marketed as an antipsychotic drug.] Because parenteral administration bypasses the first pass through the portal circulation, it results in a significantly higher serum level of the parent drug than equivalent oral dosages.
Antipsychotic drugs are generally highly protein bound (85% to 90%). Clinicians have traditionally been cautioned when concomitantly treating patients with other medications that are highly protein bound (e.g., warfarin, digoxin) because of the expectation that displacement and competition for these binding sites could increase concentrations of free or unbound antipsychotics and other drugs. Antipsychotics are also highly lipophilic; thus, they readily cross the blood-brain barrier and attain high concentrations in the brain. Indeed, concentrations in the brain appear to be greater than those in blood. Given their high degree of protein and tissue binding, these drugs are not removed efficiently by dialysis.
Metabolism and Elimination
Many antipsychotic drugs are metabolized in the liver to demethylated and hydroxylated forms. These are more water soluble than the parent compounds and thus more readily excreted by the kidneys. The hydroxylated metabolites often are further metabolized by conjugation with glucuronic acid. Many of the hydroxyl and desmethyl metabolites of phenothiazines are active as dopamine receptor antagonists. The hydroxyl metabolite of the butyrophenone antipsychotic drug haloperidol (hydroxyhaloperidol) does not appear to be active. The 9-hydroxyrisperidone metabolite of risperidone, paliperidone (Invega), is marketed as a separate antipsychotic. Much remains unknown about the metabolites of other chemical classes of antipsychotics.
The elimination half-life of most of the first-generation antipsychotic drugs is 18 to 40 hours, but numerous factors, such as genetically determined metabolic rates, age, and the coadministration of other hepatically metabolized drugs, affect the half-life to such a degree that plasma levels may vary among individuals by 10-to 20-fold. The elimination half-life of several of the second-generation antipsychotics (quetiapine, ziprasidone, risperidone) is less than 12 hours, whereas the elimination half-life of aripiprazole is approximately 3 days.
Long-Acting Preparations
Long-acting preparations of haloperidol and fluphenazine are available in which the active drug is esterified to a lipid side chain. The drug is given as an i.m. injection in an oily vehicle (sesame oil) that slows absorption. The only first-generation preparations currently available in the United States are the decanoate ester of fluphenazine and the decanoate ester of haloperidol. Fluphenazine decanoate has a half-life of 7 to 10 days, allowing administration approximately every 2 weeks, although some patients may tolerate longer spacing of the injections. Haloperidol decanoate has a longer half-life, allowing dosing intervals of 3 to 6 weeks, depending on the individual. Long-acting preparations of second-generation drugs are in development for several drugs, and risperidone (Consta) is available in the United States and Europe. This preparation, using microspheres as the vehicle, has a half-life of 7 days, allowing for injections every 2 weeks. A long-acting preparation of olanzapine is under review by the FDA (though will likely be unapproved because of problematic sedation), and a long-acting preparation of paliperidone is being developed.
Blood Levels
Given the marked interindividual differences in plasma levels produced by a given oral dose and the concerns about the consequences of noncompliance among psychotic patients, it would be useful to have some objective measure of drug level to
aid in optimizing efficacy and clinical improvement. Specifically, it has been hoped that a range of therapeutic blood levels could be determined for the various antipsychotic drugs. Unfortunately, the measurement of blood levels by various chromatographic techniques and mass spectroscopy has not correlated that well with clinical response, though there is a role for monitoring olanzapine and clozapine. For olanzapine, there is preliminary evidence of a relationship between clinical outcomes and plasma concentrations. A therapeutic range of 20 to 50 ng/mL has been suggested for olanzapine. For clozapine, there appears to be a threshold concentration for response, at approximately 350 ng/mL, although one study found a range of 200 to 300 ng/mL as effective as plasma concentrations above 350 ng/mL. Higher concentrations are associated with more toxicity and electroencephalographic changes. Some antipsychotic drugs have so many active metabolites (e.g., thioridazine) that measurement to assess dose-response relationships is impractical. Thus, clinical observation and documentation of specific symptom changes over time remain the mainstays of assessment of drug efficacy, although plasma levels can still be useful to determine extreme concentrations (close to zero or very high), suggesting either nonadherence or unusual metabolism.
MECHANISM OF ACTION
The therapeutic mechanism of action of the antipsychotic drugs is only partly understood. The first-generation (e.g., haloperidol-like) antipsychotic drugs and the second-generation drugs risperidone, paliperidone, ziprasidone, and aripiprazole are all potent antagonists of D
2 dopamine receptors. On the other hand, clozapine and quetiapine are weak D
2 antagonists, and by positron emission tomography, they show significantly lower levels of D
2 receptor occupancy at effective doses, compared with the haloperidol-like drugs. A common property of second-generation antipsychotic drugs is the ability to block serotonin 5-HT
2A receptors. Olanzapine, risperidone, aripiprazole, and ziprasidone do so with high affinity; quetiapine has relatively lower affinity for all its receptor targets (
Table 2.1). The second-generation drugs interact with a variety of other serotonin receptors but not with any obvious pattern. All but quetiapine have high affinity for 5-HT
2C receptors; all but risperidone have high affinity for 5-HT
6 receptors; risperidone has a particularly high affinity for the 5-HT
7 receptor. All of the second-generation antipsychotics antagonize α
1-adrenergic receptors and histamine H
1 receptors, which may contribute to side effects. Both clozapine and olanzapine are strongly anticholinergic.
This complex picture of binding properties makes it difficult to pinpoint the mechanism of action. D2 receptor antagonism correlates well with both efficacy and EPS liability for the first-generation (haloperidol-like) antipsychotic drugs. In addition, blockade of 5-HT2A receptors appears to correlate with diminished EPS liability. Drugs that antagonize only 5-HT2A receptors, however, do not have antipsychotic properties. Given that clozapine exhibits a high ratio of D4 antagonism to D2 antagonism, there was much excitement about a possible role for D4 antagonists as antipsychotic drugs. However, relatively selective D4 antagonists, as well as a mixed D4 and 5-HT2A antagonist (fananserin), have not shown antipsychotic efficacy.
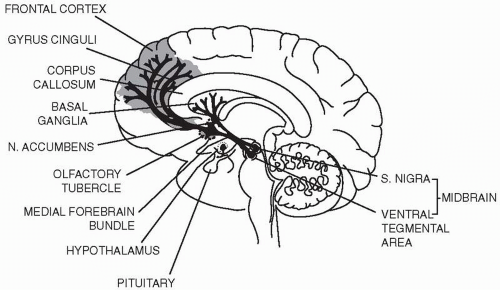
To date it appears that blockade of D
2 receptors in the targets of mesolimbic and mesocortical dopamine projections from the ventral tegmental area is responsible for initiating the therapeutic actions of first-generation antipsychotic drugs and likely for the clozapine-like drugs that exhibit lower D
2 affinity. D
2 blockade in the striatum is responsible for the extrapyramidal effects of the typical antipsychotic drugs (
Fig. 2.1). In addition to these midbrain dopamine systems, there is a dopamine projection in the tuberoinfundibular system of the hypothalamus. In this system, dopamine acts as an inhibitor of the synthesis and release of prolactin by pituitary lactotrophs. By antagonizing dopamine in this system, antipsychotic
drugs with strong D
2 antagonist properties often produce hyperprolactinemia. The key to the increased efficacy of clozapine, despite low affinity for the dopamine D
2 receptors, remains unknown.
The full therapeutic effects of antipsychotic drugs take weeks to accrue (similar to the antidepressants) and are far slower than the time required to block central nervous system (CNS) receptors or, in most cases, to achieve steady-state plasma levels of the drug. Similarly, behavioral effects in patients can last long after serum levels are no longer detectable. Such observations suggest that the therapeutic response to antipsychotic drugs is a secondary or adaptive response to receptor blockade with a time course characterized by slower onset and offset than would be predicted by serum or even brain levels. (D2 receptor occupancy in the human brain may now be measured experimentally from positron emission tomography.) In as much as some initially responsive patients relapse even with apparently adequate serum levels of drugs, other types of adaptations may occur in the brain, reflecting such factors as primary alterations in the disease process, changes in the psychosocial circumstances of the patient’s life, intercurrent psychiatric or physical illness, or drug tolerance. The therapeutically relevant delayed-onset neurobiologic effects of antipsychotic drugs remain unknown but are believed to reflect drug-activated changes in gene expression, protein synthesis, and subsequent synaptic reorganization. However, there is clearly an early response to antipsychotics as well, and acutely ill patient can show benefit after a few doses. This suggests another hypothesis for the apparent delayed response to antipsychotics. Perhaps once D2 is sufficiently blocked, psychosis starts to recede. The full resolution of psychosis, however, occurs only if patients deconstruct their psychotic worldview. This psychological process of course takes time and might have led to the somewhat incorrect view that antipsychotics take weeks to show an effect.
In addition to their effects on dopamine receptors, antipsychotic drugs may cause side effects by binding to a variety of other neurotransmitter receptors. For example, low-potency conventional antipsychotic drugs are potent antagonists of muscarinic cholinergic receptors with highest relative affinity for thioridazine
followed by chlorpromazine. Among the second-generation drugs, clozapine and olanzapine also have substantial
anticholinergic potency. As a result, these drugs can produce side effects such as dry mouth and constipation.
Postural hypotension is produced by antagonism of α1-adrenergic receptors. Antipsychotic drugs with substantial affinity for this receptor include many first-generation compounds, especially chlorpromazine and thioridazine. Haloperidol, however, has very little propensity for antagonism of α1-adrenergic receptors. Several of the second-generation antipsychotics, especially risperidone and quetiapine, have substantial affinity for α1-adrenergic receptors and may cause orthostatic hypotension.
Sedation appears to result from antagonism of several neurotransmitter receptors, including α1-adrenergic, muscarinic, and histamine H1 receptors. Because of substantial affinity for these receptor types, low-potency antipsychotics, such as chlorpromazine and thioridazine, and all the second-generation drugs can be sedating (particularly clozapine, olanzapine, and quetiapine). Weight gain is a significant consequence of certain second-generation antipsychotics, particularly for clozapine and olanzapine. Weight gain is less problematic with ziprasidone and aripiprazole. The mechanism of weight gain is unknown and may be multifactorial. Blockade of histamine H1 receptors and increases in plasma leptin or insulin levels are currently being investigated as possible mechanisms. New-onset diabetes or worsening of existing diabetes may occur with clozapine or olanzapine and sometimes with other antipsychotic drugs. In addition, many antipsychotic drugs block certain calcium channels on neurons, cardiac muscle, and smooth muscle.
Glutamate receptors may also be involved in antipsychotics’ mechanism of action, though their involvement remains poorly understood. The hypothesized contribution of glutamate to the symptoms of psychosis is largely derived from pharmacologic studies that find that the N-methyl-D-aspartate glutamate antagonist phenylcyclidine induces a clinical syndrome similar to schizophrenia and may exacerbate psychosis in patients with schizophrenia. Because the N-methyl-D-aspartate receptor requires the presence of glutamate and the co-agonist glycine for effective gating, studies have been done with glycine agonists such as D-cycloserine, though these have been disappointing. On the other hand, a recent study of a drug under development finds that a drug (LY 404039) that is an agonist at metabotropic glutamate receptors, but does not antagonize dopamine receptors, was clinically superior to placebo for treating schizophrenia. This drug, however, was less effective than olanzapine in this trial. This promising study may open a new line of investigation into drugs that do not directly affect dopamine receptors.
LONG-TERM TREATMENT OF SCHIZOPHRENIA
Many studies have proven that long-term treatment with antipsychotic drugs increases the time between exacerbations among schizophrenic patients who respond to short-term treatment. The relapse rate for schizophrenic patients who are not on maintenance antipsychotic drugs may be as high as 50% at 6 months and 65% to 80% at 12 months, whereas for those maintained on antipsychotic drugs, the relapse rate may be 10% to 15% at 6 months and no higher than 25% at 12 months. Given the disorder’s morbidity, most patients with well-diagnosed schizophrenia will have net benefit from long-term maintenance treatment. Intermittent treatment is problematic in patients with chronic psychosis but might be a possibility in selected patients who can recognize the beginning of a new episode. Because long-term treatment with typical antipsychotic drugs brings with it the risk of TD, the clinician should carefully consider the risks and benefits of using the older drugs over time with each patient.
Some patients with refractory symptoms have been treated with high antipsychotic drug doses (more than the equivalent of 20 mg per day of haloperidol),
often more reflective of physician frustration than appropriate therapy. There is
no evidence of extra benefit at very high doses of antipsychotic drugs, although side effects are clearly worsened. Currently, for patients who have failed to respond to adequate doses of several antipsychotic drugs, including atypical drugs, a trial of clozapine is indicated. As described previously, clozapine is the only antipsychotic drug that has been convincingly shown to be effective for substantial numbers of patients with schizophrenia who have proved refractory to other antipsychotic drugs.
THERAPEUTIC USE
Choosing an Antipsychotic Drug
The first-generation antipsychotics provided a breakthrough for previously untreatable psychosis. They, however, presented significant acute neurological side effects and often left patients with the telltale sign of TD. With the second-generation antipsychotics, the problem of neurological toxicity appeared to have been resolved, and these drugs even appeared to be somewhat more effective, but over time it appears that the field’s enthusiasm has been premature: second-generation antipsychotics are probably only as effective as first-generation antipsychotics. Moreover, some second-generation antipsychotics can cause extrapyramidal side effects, and others often lead to weight gain, hyperlipidemia, and insulin resistance. Hence, despite the near-total switch to the second-generation antipsychotics and the tremendous increase in costs, the overall advantages of the second-generation to the first-generation antipsychotics appear minor.
The second-generation antipsychotics have a lower incidence of EPS symptoms such as dystonia, tremor, stiffness and rigidity, akathisia, and altered affect, as well as greatly diminished risk of neuroleptic malignant syndrome (NMS) and TD. Second-generation antipsychotics are the most commonly used antipsychotics as the initial drug for a first episode, though this practice may be supported by the widespread belief of their superiority rather than by solid evidence confirming their superiority. Indeed, two large-scale effectiveness studies, one in the United States and one in Britain, that compared first- and second-generation antipsychotics found minor effectiveness differences between older and newer antipsychotics in real-world settings. In the British study, second-generation antipsychotics were no better than first-generation antipsychotics over the 1-year study period, and patients failed to express a preference between drug classes. In the U.S. study, perphenazine appeared marginally less effective than olanzapine, and similar to risperidone, ziprasidone, and quetiapine. Many suggest that the metabolic burden of olanzapine limits its long-term use. The wide uptake of these drugs may have been due to marketing and expectations of clozapine-type results rather than to true clinical advantages.
Although clozapine heralded a new era in the pharmacotherapy of psychosis, particularly with neuroleptic-resistant patients and other subpopulations (e.g., patients with Parkinson’s disease), the risk of agranulocytosis complicated the use of this agent. The efficacy of clozapine for both positive and negative symptoms in acute and chronic schizophrenia is an additional feature of this compound and demonstrated that the effective treatment of psychoses was not inexorably linked with EPS. This observation prompted the search for similar compounds without the hematologic side effect.
All of the compounds approved as antipsychotic drugs in the United States are efficacious. Clozapine is more effective than the others in the treatment of schizophrenia. The second-generation antipsychotic drugs differ only in their potency (the dosage needed to produce the desired effect) and side effects. Among the older antipsychotics, drugs that are most potent tend to produce more EPS and those
that are less potent produce more sedation, postural hypotension, and anticholinergic effects (
Table 2.2). For example, 8 mg of haloperidol and 400 mg of chlorpromazine are equivalent regarding antipsychotic efficacy; however, the patient receiving haloperidol would be more likely to develop EPS, and the patient taking chlorpromazine would be more likely to feel sedated and to develop postural hypotension. The first-generation compounds are more likely to induce EPS than the second-generation compounds, though all of the second-generation drugs, particularly risperidone and aripiprazole, can cause EPS. In addition, they
all can cause sedation and postural hypotension and, except possibly ziprasidone and aripiprazole, can cause weight gain (particularly clozapine and olanzapine). Among the newer agents, risperidone and paliperidone are especially likely to elevate plasma prolactin, whereas the other second-generation agents are rarely associated with prolactinemia. Aripiprazole can even lower prolactin levels. With the exception of clozapine in treatment-refractory schizophrenia, because there is no established difference in the therapeutic effectiveness of these drugs, side-effect profiles are a central consideration when starting a patient on antipsychotic treatment.
A patient who has responded well to a particular psychotropic drug in the past is likely to do well on the same drug again. On the other hand, even if a patient has no history of severe EPS or other troublesome side effects with a particular first generation antipsychotic, the physician should consider initiating therapy with a newer compound that would have considerably less or no EPS or risk of TD over the long term. In considering the acute advantages offered by more sedating agents (e.g., for the young patient with severe insomnia), the clinician could also consider the acute use of a less sedating compound combined with temporary use of a benzodiazepine (e.g., lorazepam or clonazepam) to achieve sedation so that when the acute episode passes, the sedation can be dissected from the treatment by stopping the hypnotic medication if desired. For example, although olanzapine may allow for greater sedation in acute treatment than ziprasidone or aripiprazole, long-term use of olanzapine may lead to daytime sedation and weight gain, whereas ziprasidone or aripiprazole may be better tolerated over the long term.
For certain groups of patients, toxic effects determine drugs to avoid. Common examples are as follows: (a) patients with cardiac conduction problems should avoid pimozide; (b) patients with Parkinson’s disease should avoid high-potency antipsychotics; (c) patients with diabetes should generally avoid clozapine or olanzapine; and (d) clozapine should be administered only to patients who are adequately compliant so that they will comply with frequent blood drawing.
Use of Antipsychotic Drugs
Because antipsychotic drugs can produce striking changes in the thinking, language, and behavior (including motor behavior) of the patients who receive them, a thorough examination of physical and mental status should be performed before initiating therapy. In psychotic disorders, the mental status may fluctuate markedly; thus, on occasion a brief initial period of drug-free observation may be helpful for the hospitalized patient when the diagnosis is unclear. A reliable history must always supplement the mental status examination. In all cases, but especially when the diagnosis is unclear, it is important to objectively rate target symptoms that can be monitored throughout the treatment with antipsychotic drugs. The physician must have a clear notion of the symptoms that are likely to respond specifically to antipsychotic drugs and the symptoms (e.g., anxiety) that may be better treated by other classes of drugs.
The latency of response of psychotic symptoms such as delusions, hallucinations, and bizarre behavior is usually several days, with nonspecific sedation occurring more rapidly. The full benefit in antipsychotic-responsive disorders usually takes at least 2 to 6 weeks. Unfortunately, physicians are often impatient in
treating psychoses given the level of patient and family distress and the pressure of disruptive behavior on the inpatient unit.
Thus, premature dosage increases occur, and when symptoms finally remit, the time course of improvement is often mistaken for a requirement for high doses of antipsychotic drugs. Aripiprazole has a half-life of 3 days, and dose adjustments during a brief hospitalization would not even be apparent until after hospital discharge. If dangerous behavior must be rapidly controlled, the physician may choose to increase the antipsychotic drug dosage temporarily to exploit its nonspecific sedative properties or perhaps more wisely choose to use an adjunctive benzodiazepine as needed. Valproate can be added to reduce agitation in acute psychosis, although it has no benefit in the long run for patients with schizophrenia. In either case, once the acute symptoms subside, extra medications administered for sedation should be tapered and an optimal antipsychotic drug dosage established.